Sandbox Reserved 1451
From Proteopedia
| Line 17: | Line 17: | ||
then the signal can be transmitted to the occipital lobe. Over 120 point mutations to rhodopsin | then the signal can be transmitted to the occipital lobe. Over 120 point mutations to rhodopsin | ||
have been identified which can lead to night blindness and more several visual problems<ref name="Article1">PMID:22266727</ref>. | have been identified which can lead to night blindness and more several visual problems<ref name="Article1">PMID:22266727</ref>. | ||
| + | == Function == | ||
| + | Rhodopsin performs two functions. One function is to bind retinal. Rhodopsin is a protein that is essential for vision, especially in dim light. The photoreceptors in the retina that contain rhodopsin are rods. Rhodopsin is attached to 11-cis retinal which becomes excited by a photon of light and isomerizes to become all-trans conformation<ref name="Article4">PMID:29042326</ref>. This excitation activates rhodopsin and leads to depolarizing of neurons. The depolarizing of neurons is how the image is transmitted to the brain<ref name="Article2">“RHO Gene - Genetics Home Reference.” U.S. National Library of Medicine, National Institutes of Health, 11AD, ghr.nlm.nih.gov/gene/RHO#.</ref>. Another function is to function as a G protein-coupled receptor. When rhodopsin is activated by light the protein couples with the G protein transducin which is the first step in the signal cascade<ref name="Article4">PMID:29042326</ref>. Rhodopsin must undergo several conformational changes before being able to bind transducin<ref name="Article3">PMID:21352497</ref>. Rhodopsin is initially converted to metarhodopsin II which is the active form of rhodopsin. Once the protein is active then metarhodopsin binds the G-protein tranducin and the GDP is exchanged for GTP. The G protein subunits dissociate and causes a decrease in cytosolic cGMP. The decrease in cGMP also causes the closing of calcium channels. When calcium concentrations drop the photoreceptors become hyperpolarized and this ensures the action potential is sent onward toward the brain<ref name="Article5">PMID:12402507</ref>. Photoreceptors are unable to be immediately restimulated, rhodopsin kinase phosphorylates the protein on serine and threonine residues which leads to the binding of arrestin. Arrestin prevents rhodopsin from interacting with tranducin again, thus preventing restimulation of the protein. Arrestin is released from the protein via phospotase A which dephosphorylates rhodopsin<ref name="Article5">PMID:12402507</ref>. | ||
Revision as of 19:45, 30 April 2018
>
| This Sandbox is Reserved from Jan 22 through May 22, 2018 for use in the course Biochemistry II taught by Jason Telford at the Maryville University, St. Louis, Missouri, USA. This reservation includes Sandbox Reserved 1446 through Sandbox Reserved 1455. |
To get started:
More help: Help:Editing |
|
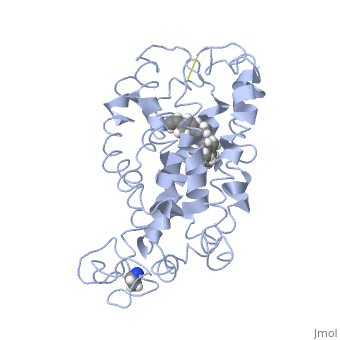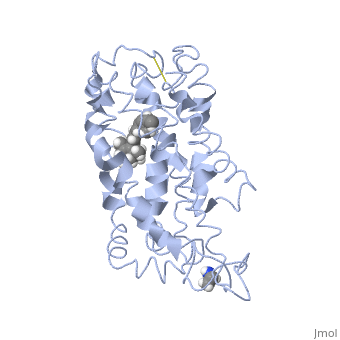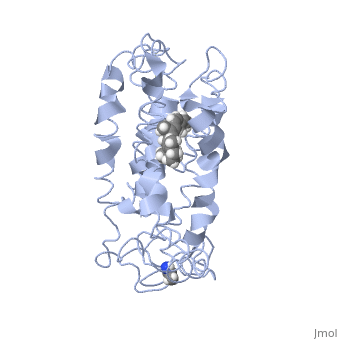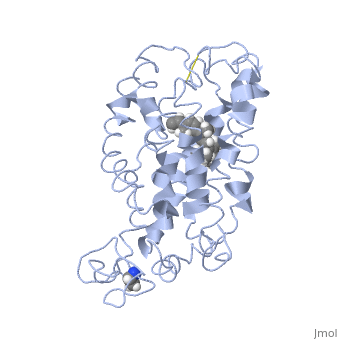
Rhodopsin
Rhodopsin is a member of the G-protein coupled receptor (GPCR) family. Rhodopsin is the common GPCR structure used to understand functionality of G-protein coupled receptors. Rhodopsin is commonly found in the photoreceptors in the retina, specifically in the rod photoreceptors and become activated by photons of light. Rhodopsin contains a chromophore (compound that absorbs light), specifically 11-cis-retinal, which when active recruites G proteins to transmit a signaling cascade in neural impulses to the gray matter of the occipital lobe. Once the receptor has been activated, a new rhodopsin needs to be regenerated. Rhodopsin is located in the rod outer segment (ROS) which consists of stacked disks enclosed by a membrane. The entire family of GPCR’s have the common structure of seven alpha-helices across membranes. Rhodopsin’s structure changes upon photoactivation. The sixth helix bends away from the seventh creating a pocket that allows for binding of a G protein. A salt bridge covers this pocket until the helices shift away from one another. Once the G protein has bound then the signal can be transmitted to the occipital lobe. Over 120 point mutations to rhodopsin have been identified which can lead to night blindness and more several visual problems[1].
Function
Rhodopsin performs two functions. One function is to bind retinal. Rhodopsin is a protein that is essential for vision, especially in dim light. The photoreceptors in the retina that contain rhodopsin are rods. Rhodopsin is attached to 11-cis retinal which becomes excited by a photon of light and isomerizes to become all-trans conformation[2]. This excitation activates rhodopsin and leads to depolarizing of neurons. The depolarizing of neurons is how the image is transmitted to the brain[3]. Another function is to function as a G protein-coupled receptor. When rhodopsin is activated by light the protein couples with the G protein transducin which is the first step in the signal cascade[2]. Rhodopsin must undergo several conformational changes before being able to bind transducin[4]. Rhodopsin is initially converted to metarhodopsin II which is the active form of rhodopsin. Once the protein is active then metarhodopsin binds the G-protein tranducin and the GDP is exchanged for GTP. The G protein subunits dissociate and causes a decrease in cytosolic cGMP. The decrease in cGMP also causes the closing of calcium channels. When calcium concentrations drop the photoreceptors become hyperpolarized and this ensures the action potential is sent onward toward the brain[5]. Photoreceptors are unable to be immediately restimulated, rhodopsin kinase phosphorylates the protein on serine and threonine residues which leads to the binding of arrestin. Arrestin prevents rhodopsin from interacting with tranducin again, thus preventing restimulation of the protein. Arrestin is released from the protein via phospotase A which dephosphorylates rhodopsin[5].