This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
User:Patrick Wiencek/AHNAK
From Proteopedia
(Difference between revisions)
| Line 61: | Line 61: | ||
=== Cell signaling === | === Cell signaling === | ||
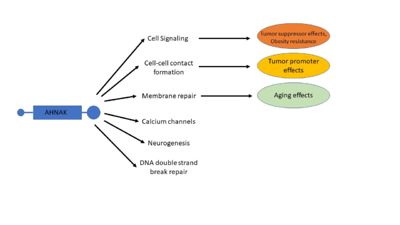
| - | The central repetitive domain of AHNAK has been shown binding and activating the signaling proteins PKCα and PLCγ < | + | The central repetitive domain of AHNAK has been shown binding and activating the signaling proteins PKCα and PLCγ <ref name="a19" /><ref name="a20" />. This activation has been demonstrated as having a downstream activating effect on the RAF/MEK/ERK pathway, which in turn regulates gene expression <ref name="a19" /><ref name="a20" /><sup>[24]</sup>. The central repetitive domain of AHNAK has also been shown to play a role in the TGFβ and Smad signaling pathway <ref name="a21" />. AHNAK can interact with and translocate regulatory-Smad proteins 1-3 to the nucleus. This translocation increases the binding of phosphor-Smad3 to the c-Myc promoter, resulting in decreased c-Myc expression and in turn less cell proliferation. AHNAK overexpression in mouse fibroblast cells resulted in an accumulation of cells in the G0 and G1 phases of the cell cycle, indicating cell cycle arrest <ref name="a21" />. |
=== Neurogenesis === | === Neurogenesis === | ||
Revision as of 20:36, 4 May 2018
AHNAK
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 Davis TA, Loos B, Engelbrecht AM. AHNAK: the giant jack of all trades. Cell Signal. 2014 Dec;26(12):2683-93. doi: 10.1016/j.cellsig.2014.08.017. Epub, 2014 Aug 27. PMID:25172424 doi:http://dx.doi.org/10.1016/j.cellsig.2014.08.017
- ↑ 2.0 2.1 2.2 Hashimoto T, Amagai M, Parry DA, Dixon TW, Tsukita S, Tsukita S, Miki K, Sakai K, Inokuchi Y, Kudoh J, et al.. Desmoyokin, a 680 kDa keratinocyte plasma membrane-associated protein, is homologous to the protein encoded by human gene AHNAK. J Cell Sci. 1993 Jun;105 ( Pt 2):275-86. PMID:8408266
- ↑ Chen B, Wang J, Dai D, Zhou Q, Guo X, Tian Z, Huang X, Yang L, Tang H, Xie X. AHNAK suppresses tumour proliferation and invasion by targeting multiple pathways in triple-negative breast cancer. J Exp Clin Cancer Res. 2017 May 12;36(1):65. doi: 10.1186/s13046-017-0522-4. PMID:28494797 doi:http://dx.doi.org/10.1186/s13046-017-0522-4
- ↑ Zhao Z, Xiao S, Yuan X, Yuan J, Zhang C, Li H, Su J, Wang X, Liu Q. AHNAK as a Prognosis Factor Suppresses the Tumor Progression in Glioma. J Cancer. 2017 Aug 25;8(15):2924-2932. doi: 10.7150/jca.20277. eCollection 2017. PMID:28928883 doi:http://dx.doi.org/10.7150/jca.20277
- ↑ Davis T, van Niekerk G, Peres J, Prince S, Loos B, Engelbrecht AM. Doxorubicin resistance in breast cancer: A novel role for the human protein AHNAK. Biochem Pharmacol. 2018 Feb;148:174-183. doi: 10.1016/j.bcp.2018.01.012. Epub, 2018 Jan 5. PMID:29309757 doi:http://dx.doi.org/10.1016/j.bcp.2018.01.012
- ↑ 6.0 6.1 Sussman J, Stokoe D, Ossina N, Shtivelman E. Protein kinase B phosphorylates AHNAK and regulates its subcellular localization. J Cell Biol. 2001 Sep 3;154(5):1019-30. doi: 10.1083/jcb.200105121. PMID:11535620 doi:http://dx.doi.org/10.1083/jcb.200105121
- ↑ 7.0 7.1 Benaud C, Gentil BJ, Assard N, Court M, Garin J, Delphin C, Baudier J. AHNAK interaction with the annexin 2/S100A10 complex regulates cell membrane cytoarchitecture. J Cell Biol. 2004 Jan 5;164(1):133-44. doi: 10.1083/jcb.200307098. Epub 2003 Dec , 29. PMID:14699089 doi:http://dx.doi.org/10.1083/jcb.200307098
- ↑ 8.0 8.1 8.2 Shtivelman E, Cohen FE, Bishop JM. A human gene (AHNAK) encoding an unusually large protein with a 1.2-microns polyionic rod structure. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5472-6. PMID:1608957
- ↑ 9.0 9.1 Cell atlas - AHNAK - The Human Protein Atlas. Available at: http://www.proteinatlas.org/ENSG00000124942-AHNAK/cell. (Accessed: 30th April 2018)
- ↑ Komuro A, Masuda Y, Kobayashi K, Babbitt R, Gunel M, Flavell RA, Marchesi VT. The AHNAKs are a class of giant propeller-like proteins that associate with calcium channel proteins of cardiomyocytes and other cells. Proc Natl Acad Sci U S A. 2004 Mar 23;101(12):4053-8. doi:, 10.1073/pnas.0308619101. Epub 2004 Mar 8. PMID:15007166 doi:http://dx.doi.org/10.1073/pnas.0308619101
- ↑ Lee HJ, Zheng JJ. PDZ domains and their binding partners: structure, specificity, and modification. Cell Commun Signal. 2010 May 28;8:8. doi: 10.1186/1478-811X-8-8. PMID:20509869 doi:http://dx.doi.org/10.1186/1478-811X-8-8
- ↑ 12.0 12.1 de Morree A, Droog M, Grand Moursel L, Bisschop IJ, Impagliazzo A, Frants RR, Klooster R, van der Maarel SM. Self-regulated alternative splicing at the AHNAK locus. FASEB J. 2012 Jan;26(1):93-103. doi: 10.1096/fj.11-187971. Epub 2011 Sep 22. PMID:21940993 doi:http://dx.doi.org/10.1096/fj.11-187971
- ↑ Hohaus A, Person V, Behlke J, Schaper J, Morano I, Haase H. The carboxyl-terminal region of ahnak provides a link between cardiac L-type Ca2+ channels and the actin-based cytoskeleton. FASEB J. 2002 Aug;16(10):1205-16. doi: 10.1096/fj.01-0855com. PMID:12153988 doi:http://dx.doi.org/10.1096/fj.01-0855com
- ↑ Huang, Y. et al. Calpain 3 is a modulator of the dysferlin protein complex in skeletal muscle. Hum. Mol. Genet. 17, 1855–1866 (2008).
- ↑ Huang, Y. et al. AHNAK, a novel component of the dysferlin protein complex, redistributes to the cytoplasm with dysferlin during skeletal muscle regeneration. FASEB J. 21, 732–742 (2006).
- ↑ Stiff T, Shtivelman E, Jeggo P, Kysela B. AHNAK interacts with the DNA ligase IV-XRCC4 complex and stimulates DNA ligase IV-mediated double-stranded ligation. DNA Repair (Amst). 2004 Mar 4;3(3):245-56. doi: 10.1016/j.dnarep.2003.11.001. PMID:15177040 doi:http://dx.doi.org/10.1016/j.dnarep.2003.11.001
- ↑ EMBOSS Needle < Pairwise Sequence Alignment < EMBL-EBI. Available at: https://www.ebi.ac.uk/Tools/psa/emboss_needle/. (Accessed: 2nd May 2018)
- ↑ AHNAK - Neuroblast differentiation-associated protein AHNAK - Homo sapiens (Human) - AHNAK gene & protein. Available at: https://www.uniprot.org/uniprot/Q09666#ptm_processing. (Accessed: 1st May 2018)
- ↑ 19.0 19.1 19.2 Lee IH, Lim HJ, Yoon S, Seong JK, Bae DS, Rhee SG, Bae YS. Ahnak protein activates protein kinase C (PKC) through dissociation of the PKC-protein phosphatase 2A complex. J Biol Chem. 2008 Mar 7;283(10):6312-20. doi: 10.1074/jbc.M706878200. Epub 2008, Jan 3. PMID:18174170 doi:http://dx.doi.org/10.1074/jbc.M706878200
- ↑ 20.0 20.1 20.2 Sekiya F, Bae YS, Jhon DY, Hwang SC, Rhee SG. AHNAK, a protein that binds and activates phospholipase C-gamma1 in the presence of arachidonic acid. J Biol Chem. 1999 May 14;274(20):13900-7. PMID:10318799
- ↑ 21.0 21.1 21.2 Lee IH, Sohn M, Lim HJ, Yoon S, Oh H, Shin S, Shin JH, Oh SH, Kim J, Lee DK, Noh DY, Bae DS, Seong JK, Bae YS. Ahnak functions as a tumor suppressor via modulation of TGFbeta/Smad signaling pathway. Oncogene. 2014 Sep 18;33(38):4675-84. doi: 10.1038/onc.2014.69. Epub 2014 Mar 24. PMID:24662814 doi:http://dx.doi.org/10.1038/onc.2014.69
- ↑ Grieve AG, Moss SE, Hayes MJ. Annexin A2 at the interface of actin and membrane dynamics: a focus on its roles in endocytosis and cell polarization. Int J Cell Biol. 2012;2012:852430. doi: 10.1155/2012/852430. Epub 2012 Feb 22. PMID:22505935 doi:http://dx.doi.org/10.1155/2012/852430
- ↑ Rezvanpour A, Santamaria-Kisiel L, Shaw GS. The S100A10-annexin A2 complex provides a novel asymmetric platform for membrane repair. J Biol Chem. 2011 Nov 18;286(46):40174-83. doi: 10.1074/jbc.M111.244038. Epub, 2011 Sep 26. PMID:21949189 doi:http://dx.doi.org/10.1074/jbc.M111.244038