User:Patrick Wiencek/AHNAK
From Proteopedia
(Difference between revisions)
| Line 69: | Line 69: | ||
=== Cell-Cell contact formation === | === Cell-Cell contact formation === | ||
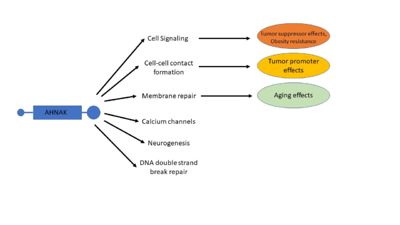
| - | In addition to phosphorylation by PKB, AHNAK localization in epithelial cells depends on cell confluency, where sub-confluent cells displayed a nuclear localization while confluent cells displayed a cytoplasmic or plasma membrane localization < | + | In addition to phosphorylation by PKB, AHNAK localization in epithelial cells depends on cell confluency, where sub-confluent cells displayed a nuclear localization while confluent cells displayed a cytoplasmic or plasma membrane localization <ref name="a6" />. When AHNAK re-localizes to the plasma membrane it complexes with actin and heterotetrameric annexin2/S100A10 <ref name="a7" />. A structural analysis of this complex reveals that both annexin2 and S100A10 are necessary for the complex to form <sup>[29]</sup>. A possible mechanism for calcium dependent cell-cell contact formation is that PKB phosphorylation of AHNAK will cause its translocation to the plasma membrane where it complexes with actin and annexin2/S100A10 <ref name="a1" /><ref name="a6" /><ref name="a7" />. |
=== Calcium channels === | === Calcium channels === | ||
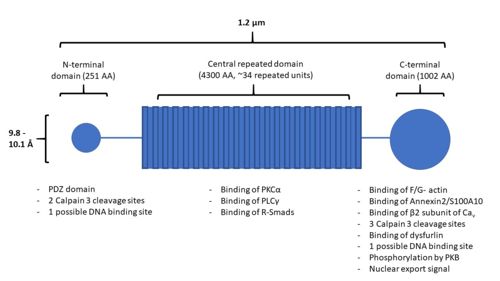
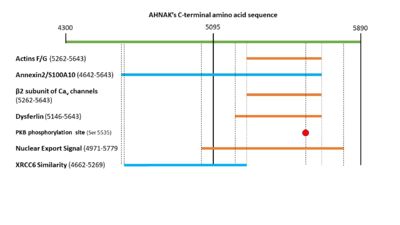
| - | AHNAK can bind the β2 subunit of L-type voltage gated calcium (Cav ) channels in cardiomyocytes < | + | AHNAK can bind the β2 subunit of L-type voltage gated calcium (Cav ) channels in cardiomyocytes <ref name="a13" />. AHNAK seems to have different effects on calcium channels and from calcium across the cited studies. This may be due to different calcium channel isoforms, or different cell types (and thus different responses to calcium) <ref name="a1" />. One hypothesis of AHNAK function with the β2 subunit is that following β-adrenergic stimulation and phosphorylation of AHNAK by PKA, AHNAK will release the β2 subunit of the Cav channel and allow normal calcium influx <sup>[31]</sup>. AHNAK was also implicated in calcium influx in CD4+ T cells and cytotoxic CD8+ effector T-cells <sup>[32,33]</sup>. Here, AHNAK null mice showed decreased calcium influx, leading experts to hypothesize that the underlying mechanism involved AHNAK assisting the β2-subunit in membrane localization <sup>[34]</sup>. |
=== Membrane repair === | === Membrane repair === | ||
| - | AHNAK is involved in the process of membrane repair through its presence in enlargeosomes, vesicles that fuse with the plasma membrane for differentiation and membrane repair <sup>[35]</sup>. AHNAK typically marks these enlargeosomes just below the plasma membrane. When stimulated with ionomycin AHNAK will label the plasma membrane, as would be expected from a membrane fusion event <sup>[35]</sup>. AHNAK co-localizes and interacts with a membrane repair protein dysferlin, which also interacts with the annexin2/S100A10 complex <sup>[ | + | AHNAK is involved in the process of membrane repair through its presence in enlargeosomes, vesicles that fuse with the plasma membrane for differentiation and membrane repair <sup>[35]</sup>. AHNAK typically marks these enlargeosomes just below the plasma membrane. When stimulated with ionomycin AHNAK will label the plasma membrane, as would be expected from a membrane fusion event <sup>[35]</sup>. AHNAK co-localizes and interacts with a membrane repair protein dysferlin, which also interacts with the annexin2/S100A10 complex <ref name="a15" /><sup>[36]</sup>. AHNAK’s interaction with S100A10 is small enough to allow it to still interact with dysferlin <sup>[23,29]</sup>. This complex may be regulated by calpain 3, a protease that has been implicated in limb girdle muscular dystrophy A2 along with dysferlin and was experimentally shown to cleave AHNAK <sup>[14]</sup>. |
=== Repair of double strand breaks === | === Repair of double strand breaks === | ||
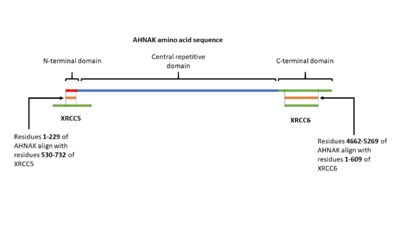
| - | In 2004, AHNAK was published interacting specifically with the DNA ligase IB-XRCC4 complex, which is involved in non-homologous end joining < | + | In 2004, AHNAK was published interacting specifically with the DNA ligase IB-XRCC4 complex, which is involved in non-homologous end joining <ref name="a16" />. This interaction is not observed with AHNAK and other DNA ligases. AHNAK was shown having a weak DNA-binding affinity by itself, but formed a more stable complex when complexed with DNA and DNA ligase. |
== '''AHNAK in Disease''' == | == '''AHNAK in Disease''' == | ||
| - | Despite initial mouse models that showed no phenotypic defects in AHNAK-null mice, AHNAK has been related to several different diseases <sup>[ | + | Despite initial mouse models that showed no phenotypic defects in AHNAK-null mice, AHNAK has been related to several different diseases <ref name="a10" /><sup>[37]</sup>. These include but are not limited to: cancer, obesity, and aging <ref name="a1" /><sup>[38-40]</sup>. |
=== Cancer === | === Cancer === | ||
| Line 90: | Line 90: | ||
AHNAK’s roles in cancer and tumor metastasis have recently become a large part of the research being done with AHNAK. Due to AHNAK’s implications in many different biological processes, AHNAK seems to promote cancer in some contexts <sup>[30,41]</sup>, and serve as a tumor suppressor in others <sup>[3,4,21]</sup>. Due to its functionality in cytoskeletal stabilization and interaction with actin filaments, AHNAK was found to be essential in actin-rich pseudopod protrusion across several different metastatic human tumor cell lines <sup>[30]</sup>. AHNAK knockdown caused these cells to retract their pseudopods and reverse the epithelial to mesenchymal transition that is necessary for cancer metastasis <sup>[30]</sup>. Similarly, significantly higher levels of AHNAK expression were detected in mesotheliomal cell lines, and migration and invasion were both decreased following AHNAK knockdown <sup>[41]</sup>. | AHNAK’s roles in cancer and tumor metastasis have recently become a large part of the research being done with AHNAK. Due to AHNAK’s implications in many different biological processes, AHNAK seems to promote cancer in some contexts <sup>[30,41]</sup>, and serve as a tumor suppressor in others <sup>[3,4,21]</sup>. Due to its functionality in cytoskeletal stabilization and interaction with actin filaments, AHNAK was found to be essential in actin-rich pseudopod protrusion across several different metastatic human tumor cell lines <sup>[30]</sup>. AHNAK knockdown caused these cells to retract their pseudopods and reverse the epithelial to mesenchymal transition that is necessary for cancer metastasis <sup>[30]</sup>. Similarly, significantly higher levels of AHNAK expression were detected in mesotheliomal cell lines, and migration and invasion were both decreased following AHNAK knockdown <sup>[41]</sup>. | ||
| - | AHNAK can also act as a tumor suppressor because of its role in the TFGβ/Smad pathway < | + | AHNAK can also act as a tumor suppressor because of its role in the TFGβ/Smad pathway <ref name="a21" />. Overexpression of AHNAK in mouse fibroblast cell resulted in increased cell-cycle arrest. Analysis of AHNAK mRNA levels in glioma demonstrated that AHNAK was down-regulated in some cell lines, and was a statistically significant prognostic factor for poor survival of glioma patients <sup>[4]</sup>. Similar results were shown in a study of AHNAK in triple-negative breast cancer, also associating AHNAK with the AMK/MAPK signaling pathway and the Wnt/β-catenin pathway <ref name="a3" />. These differing effects of AHNAK in cancer may involve its regulation via TGFβ, which has both tumor suppressor and tumor promotor roles <ref name="a1" /><sup>[42]</sup>. |
=== Obesity === | === Obesity === | ||
Revision as of 20:41, 4 May 2018
AHNAK
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 Davis TA, Loos B, Engelbrecht AM. AHNAK: the giant jack of all trades. Cell Signal. 2014 Dec;26(12):2683-93. doi: 10.1016/j.cellsig.2014.08.017. Epub, 2014 Aug 27. PMID:25172424 doi:http://dx.doi.org/10.1016/j.cellsig.2014.08.017
- ↑ 2.0 2.1 2.2 Hashimoto T, Amagai M, Parry DA, Dixon TW, Tsukita S, Tsukita S, Miki K, Sakai K, Inokuchi Y, Kudoh J, et al.. Desmoyokin, a 680 kDa keratinocyte plasma membrane-associated protein, is homologous to the protein encoded by human gene AHNAK. J Cell Sci. 1993 Jun;105 ( Pt 2):275-86. PMID:8408266
- ↑ 3.0 3.1 Chen B, Wang J, Dai D, Zhou Q, Guo X, Tian Z, Huang X, Yang L, Tang H, Xie X. AHNAK suppresses tumour proliferation and invasion by targeting multiple pathways in triple-negative breast cancer. J Exp Clin Cancer Res. 2017 May 12;36(1):65. doi: 10.1186/s13046-017-0522-4. PMID:28494797 doi:http://dx.doi.org/10.1186/s13046-017-0522-4
- ↑ Zhao Z, Xiao S, Yuan X, Yuan J, Zhang C, Li H, Su J, Wang X, Liu Q. AHNAK as a Prognosis Factor Suppresses the Tumor Progression in Glioma. J Cancer. 2017 Aug 25;8(15):2924-2932. doi: 10.7150/jca.20277. eCollection 2017. PMID:28928883 doi:http://dx.doi.org/10.7150/jca.20277
- ↑ Davis T, van Niekerk G, Peres J, Prince S, Loos B, Engelbrecht AM. Doxorubicin resistance in breast cancer: A novel role for the human protein AHNAK. Biochem Pharmacol. 2018 Feb;148:174-183. doi: 10.1016/j.bcp.2018.01.012. Epub, 2018 Jan 5. PMID:29309757 doi:http://dx.doi.org/10.1016/j.bcp.2018.01.012
- ↑ 6.0 6.1 6.2 6.3 Sussman J, Stokoe D, Ossina N, Shtivelman E. Protein kinase B phosphorylates AHNAK and regulates its subcellular localization. J Cell Biol. 2001 Sep 3;154(5):1019-30. doi: 10.1083/jcb.200105121. PMID:11535620 doi:http://dx.doi.org/10.1083/jcb.200105121
- ↑ 7.0 7.1 7.2 7.3 Benaud C, Gentil BJ, Assard N, Court M, Garin J, Delphin C, Baudier J. AHNAK interaction with the annexin 2/S100A10 complex regulates cell membrane cytoarchitecture. J Cell Biol. 2004 Jan 5;164(1):133-44. doi: 10.1083/jcb.200307098. Epub 2003 Dec , 29. PMID:14699089 doi:http://dx.doi.org/10.1083/jcb.200307098
- ↑ 8.0 8.1 8.2 Shtivelman E, Cohen FE, Bishop JM. A human gene (AHNAK) encoding an unusually large protein with a 1.2-microns polyionic rod structure. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5472-6. PMID:1608957
- ↑ 9.0 9.1 Cell atlas - AHNAK - The Human Protein Atlas. Available at: http://www.proteinatlas.org/ENSG00000124942-AHNAK/cell. (Accessed: 30th April 2018)
- ↑ 10.0 10.1 Komuro A, Masuda Y, Kobayashi K, Babbitt R, Gunel M, Flavell RA, Marchesi VT. The AHNAKs are a class of giant propeller-like proteins that associate with calcium channel proteins of cardiomyocytes and other cells. Proc Natl Acad Sci U S A. 2004 Mar 23;101(12):4053-8. doi:, 10.1073/pnas.0308619101. Epub 2004 Mar 8. PMID:15007166 doi:http://dx.doi.org/10.1073/pnas.0308619101
- ↑ Lee HJ, Zheng JJ. PDZ domains and their binding partners: structure, specificity, and modification. Cell Commun Signal. 2010 May 28;8:8. doi: 10.1186/1478-811X-8-8. PMID:20509869 doi:http://dx.doi.org/10.1186/1478-811X-8-8
- ↑ 12.0 12.1 de Morree A, Droog M, Grand Moursel L, Bisschop IJ, Impagliazzo A, Frants RR, Klooster R, van der Maarel SM. Self-regulated alternative splicing at the AHNAK locus. FASEB J. 2012 Jan;26(1):93-103. doi: 10.1096/fj.11-187971. Epub 2011 Sep 22. PMID:21940993 doi:http://dx.doi.org/10.1096/fj.11-187971
- ↑ 13.0 13.1 Hohaus A, Person V, Behlke J, Schaper J, Morano I, Haase H. The carboxyl-terminal region of ahnak provides a link between cardiac L-type Ca2+ channels and the actin-based cytoskeleton. FASEB J. 2002 Aug;16(10):1205-16. doi: 10.1096/fj.01-0855com. PMID:12153988 doi:http://dx.doi.org/10.1096/fj.01-0855com
- ↑ Huang, Y. et al. Calpain 3 is a modulator of the dysferlin protein complex in skeletal muscle. Hum. Mol. Genet. 17, 1855–1866 (2008).
- ↑ 15.0 15.1 Huang, Y. et al. AHNAK, a novel component of the dysferlin protein complex, redistributes to the cytoplasm with dysferlin during skeletal muscle regeneration. FASEB J. 21, 732–742 (2006).
- ↑ 16.0 16.1 Stiff T, Shtivelman E, Jeggo P, Kysela B. AHNAK interacts with the DNA ligase IV-XRCC4 complex and stimulates DNA ligase IV-mediated double-stranded ligation. DNA Repair (Amst). 2004 Mar 4;3(3):245-56. doi: 10.1016/j.dnarep.2003.11.001. PMID:15177040 doi:http://dx.doi.org/10.1016/j.dnarep.2003.11.001
- ↑ EMBOSS Needle < Pairwise Sequence Alignment < EMBL-EBI. Available at: https://www.ebi.ac.uk/Tools/psa/emboss_needle/. (Accessed: 2nd May 2018)
- ↑ AHNAK - Neuroblast differentiation-associated protein AHNAK - Homo sapiens (Human) - AHNAK gene & protein. Available at: https://www.uniprot.org/uniprot/Q09666#ptm_processing. (Accessed: 1st May 2018)
- ↑ 19.0 19.1 19.2 Lee IH, Lim HJ, Yoon S, Seong JK, Bae DS, Rhee SG, Bae YS. Ahnak protein activates protein kinase C (PKC) through dissociation of the PKC-protein phosphatase 2A complex. J Biol Chem. 2008 Mar 7;283(10):6312-20. doi: 10.1074/jbc.M706878200. Epub 2008, Jan 3. PMID:18174170 doi:http://dx.doi.org/10.1074/jbc.M706878200
- ↑ 20.0 20.1 20.2 Sekiya F, Bae YS, Jhon DY, Hwang SC, Rhee SG. AHNAK, a protein that binds and activates phospholipase C-gamma1 in the presence of arachidonic acid. J Biol Chem. 1999 May 14;274(20):13900-7. PMID:10318799
- ↑ 21.0 21.1 21.2 21.3 Lee IH, Sohn M, Lim HJ, Yoon S, Oh H, Shin S, Shin JH, Oh SH, Kim J, Lee DK, Noh DY, Bae DS, Seong JK, Bae YS. Ahnak functions as a tumor suppressor via modulation of TGFbeta/Smad signaling pathway. Oncogene. 2014 Sep 18;33(38):4675-84. doi: 10.1038/onc.2014.69. Epub 2014 Mar 24. PMID:24662814 doi:http://dx.doi.org/10.1038/onc.2014.69
- ↑ Grieve AG, Moss SE, Hayes MJ. Annexin A2 at the interface of actin and membrane dynamics: a focus on its roles in endocytosis and cell polarization. Int J Cell Biol. 2012;2012:852430. doi: 10.1155/2012/852430. Epub 2012 Feb 22. PMID:22505935 doi:http://dx.doi.org/10.1155/2012/852430
- ↑ Rezvanpour A, Santamaria-Kisiel L, Shaw GS. The S100A10-annexin A2 complex provides a novel asymmetric platform for membrane repair. J Biol Chem. 2011 Nov 18;286(46):40174-83. doi: 10.1074/jbc.M111.244038. Epub, 2011 Sep 26. PMID:21949189 doi:http://dx.doi.org/10.1074/jbc.M111.244038
- ↑ Chang F, Steelman LS, Lee JT, Shelton JG, Navolanic PM, Blalock WL, Franklin RA, McCubrey JA. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia. 2003 Jul;17(7):1263-93. doi: 10.1038/sj.leu.2402945. PMID:12835716 doi:http://dx.doi.org/10.1038/sj.leu.2402945