This is a default text for your page Jennifer Taylor/Sandbox 5. Click above on edit this page to modify. Be careful with the < and > signs.
You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
Background
Proteins are one of the four major macromolecules in biology. Present in nearly every living organism, proteins have a diverse set of functions ranging from regulating cell activity to catalyzing reactions. Proteins are primary made up of amino acids in the primary structure, which consists of the amino acid sequence. The amino acids are set in a particular order so that they perform a specific function, and this order is determined by the DNA gene that codes for amino acids. The secondary structure consists of and . These two structures are helpful in finding similar proteins that perform similar functions. The tertiary and and quaternary structures consist of the specific folding of the complete protein structure, which all comes togther in great importance when classifying proteins. [1]
Enzymes, proteins that catalyze reactions, are grouped into seven major classes based on amino acid sequence similarity and secondary structure proportions. Proteins in each class share unique properties that can classify them into more detailed subclasses. Protein structure and protein function are closely related. This means that identifying highly conserved sequences between two proteins increases the likelihood of discovering shared functions. In this study we attempted to compare the sequence and structure of an uncharacterized protein to that of a protein with a known function in order to understand the former protein’s function. In 2000, the Protein Structure Initiative began an attempt to solve the 3D-structures of proteins with known sequences in order to begin understanding their functions. But, in 2015, the Initiative no longer had the proper funding and stopped, successfully solving 6920 structures, but leaving many structures found without their functions classified as well. What we set out to do was choose a protein with a found structure, perform sequential, structural, and enzymatic analysis.
Bacterial Transformation, Protein Expression, and Protein Purification
First, we needed grow our protein that was inside a plasmid. The NEB bacterial transformation protocol was performed using DH5∂ E.Coli cells in order to insert the plasmid inside the bacteria cells. Next, an overnight culture was performed using to grow more bacteria, and therefore more proteins inside of the DNA. The plasmid was then purified and isolated from genomic DNA, proteins, ribosomes, and cell walls of bacteria using the Zippy miniprep protocol. After determining that T7 polymerase was the polymerase that transcribes 2QRU by computational analysis, it was necessary to use BL21(DE3) E.Coli to transform the cells because DH5∂ does not produce T7 polymerase. We then ran The NEB transformation protocol using BL21 cells and then a liquid culture was grown. With properly grown bacteria, we used the Zippy miniprep protocol was then run to measure the concentration of the plasmid. Next, we needed to determine that our plasmid successfully, purified, so we ran a SDS Page Gel that showed us the weight of our plasmid, that we previously were given by Snao Gene.
Structural Highlights
Functional Assay
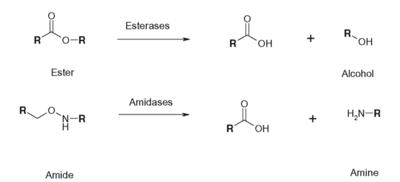
Figure 1: Esterase Reaction
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
Future Directions
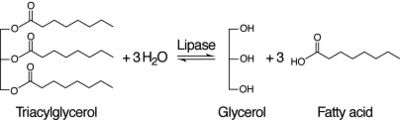
Figure 2: Lipase Reaction


