User:Jennifer Taylor/Sandbox 5
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
==Bacterial Transformation, Protein Expression, and Protein Purification== | ==Bacterial Transformation, Protein Expression, and Protein Purification== | ||
| - | First, we needed grow our protein that was inside a plasmid. The NEB bacterial transformation protocol was performed using DH5∂ E.Coli cells in order to insert the plasmid inside the bacteria cells. Next, an overnight culture was performed using to grow more bacteria, and therefore more proteins inside of the DNA. The plasmid was then purified and isolated from genomic DNA, proteins, ribosomes, and cell walls of bacteria using the Zippy miniprep protocol. After determining that T7 polymerase was the polymerase that transcribes 2QRU by computational analysis, it was necessary to use BL21(DE3) E.Coli to transform the cells because DH5∂ does not produce T7 polymerase. We then ran The NEB transformation protocol using BL21 cells and then a liquid culture was grown. With properly grown bacteria, we used the Zippy miniprep protocol was then run to measure the concentration of the plasmid. Next, we needed to determine that our plasmid successfully, purified, so we ran a SDS Page Gel that showed us the weight of our plasmid, that we previously were given by Snao Gene. | + | First, we needed grow our protein that was inside a plasmid. The NEB bacterial transformation protocol was performed using DH5∂ E.Coli cells in order to insert the plasmid inside the bacteria cells. Next, an overnight culture was performed using to grow more bacteria, and therefore more proteins inside of the DNA. The plasmid was then purified and isolated from genomic DNA, proteins, ribosomes, and cell walls of bacteria using the Zippy miniprep protocol. After determining that T7 polymerase was the polymerase that transcribes 2QRU by computational analysis, it was necessary to use BL21(DE3) E.Coli to transform the cells because DH5∂ does not produce T7 polymerase. We then ran The NEB transformation protocol using BL21 cells and then a liquid culture was grown. With properly grown bacteria, we used the Zippy miniprep protocol was then run to measure the concentration of the plasmid. Next, we needed to determine that our plasmid successfully, purified, so we ran a SDS Page Gel that showed us the weight of our plasmid, that we previously were given by Snao Gene. The Thermo Fisher Scientific Protein Purification Protocol was used to purify the protein using a His-Pur Nickel-NTA spin column and an equilibrium, a wash, and an elution buffer that we made in class. Centrifuging the column with the elution buffer, after equilibrating and washing the column, allowed protein to flow through, and give us a 2QRU sample. An SDS page gel was used to determine if the final elution samples contained the protein. The protein was successfully expressed and ready to use because its weight on the gel matched 2QRU’s weight found on SnapGene. |
| + | |||
| + | [[ImageGel.png|thumb|400 px|Figure 1: SDS Page Gel of 2QRU Purified Protein from Elution Sample]] | ||
==Structural Highlights== | ==Structural Highlights== | ||
| Line 17: | Line 19: | ||
==Functional Assay== | ==Functional Assay== | ||
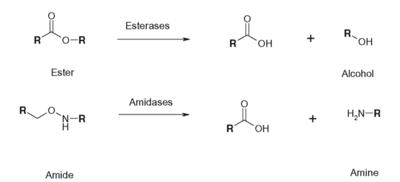
| - | [[Image:Esterase reaction.png|thumb|400 px|Figure | + | [[Image:Esterase reaction.png|thumb|400 px|Figure 2: Esterase Reaction]] |
| + | |||
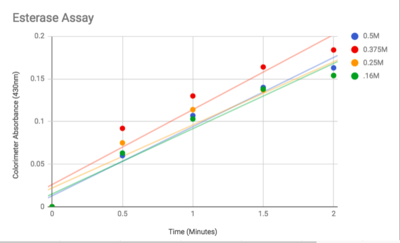
| + | An esterase activity assay using p-Nitrophenyl butyrate and a colorimeter was found online and performed with the elution samples from the Thermo Fisher Protein Purification Assay (3). Besides from the NPB with n-Heptane and the protein, a Tris (hydroxymethyl) aminomethane buffer with a pH of 8 and with 0.01% Triton was made. A blank sample with 1 mL of Tris Buffer and 6.7 uL of varied concentrations of NPB n-Heptane was vortex for 10 seconds and the put into a cuvette and blanked in the colorimeter. For every new concentration, a new blank was made. The concentrations of NPB dissolved in n-Heptane included 0.5M, 0.375M, 0.25M, 0.16M, 0.15M, 0.015M, and 0.075M. The control included 1 mL of Tris Buffer and 6.7 uL of NPB n-Heptane, which was vortexed, and then 6.7 uL of the elution sample from the Thermo Fisher Protein Purification Assay was added, transferred into a cuvette, pipetted up and down, and then the values were recorded every 30 seconds for 2 minutes. The graphs generated from this assay were important to us because we could then visualize the concentration change for all of the concentrations. A positive, increasing line would conclude that the assay was successful. | ||
| + | |||
| + | [Image:PNBReaction.png|thumb|left|400px|Figure 3: P-Nitrophenyl Reaction with Lipase]] | ||
| - | + | This experiment was important to our main goal to understand the function of 2QRU. The experiment was relatively simple and allowed us, less experienced scientists, to know why our protein was an esterase or not. | |
| - | [[Image: | + | [[Image:Esteraseactivity.png|thumb|left|400px|Figure 3: Graphed Results of our Esterase Activity]] |
| - | ==Future Directions== | + | == Results and Future Directions== |
The esterase assay proved to be successful in characterizing 2QRU as a esterase. The increasing concentration value indicated that a reaction occurred inside the cuvette containing 2QRU and p-nitrophenyl butyrate. Because p-nitrophenyl butyrate is a substrate that has specific binding for esterases, we can assume that 2QRU may be esterase because a reaction took place inside the cuvette, but more data is needed to make a confident claim. This reaction was not only evident through the OD readings but also through visible observation. Before 2QRU was added to the cuvette, the solution was a cloudy white color. However, once we added the 2QRU there was a visible color change to a bright yellow, confirming that a reaction was taking place within the cuvette. | The esterase assay proved to be successful in characterizing 2QRU as a esterase. The increasing concentration value indicated that a reaction occurred inside the cuvette containing 2QRU and p-nitrophenyl butyrate. Because p-nitrophenyl butyrate is a substrate that has specific binding for esterases, we can assume that 2QRU may be esterase because a reaction took place inside the cuvette, but more data is needed to make a confident claim. This reaction was not only evident through the OD readings but also through visible observation. Before 2QRU was added to the cuvette, the solution was a cloudy white color. However, once we added the 2QRU there was a visible color change to a bright yellow, confirming that a reaction was taking place within the cuvette. | ||
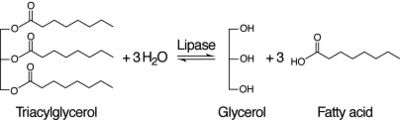
After coming to the conclusion that 2QRU is an esterase, our next research activity would be to test if 2QRU is also a lipase. Three students from The Pingry School in New Jersey performed a lipase assay with 2QRU using nitrophenyl palmitate rather than p-nitrophenyl butyrate to determine if 2QRU is a lipase. Nitrophenyl palmitate has (CH2)14 side chain than nitrophenyl butyrate, so the protein has to act on a bigger substrate. If this assay works for us, then would be able to conclude that 2QRU is also a lipase. Another avenue to explore after confirming 2QRU is a lipase is mutagenesis. If we mutated the catalytic triad responsible for the reaction with nitrophenyl palmitate and ran the lipase assay again, we should see no reaction occur in the cuvette. We can use this to confirm that 2QRU is a lipase. The same thing can be done to confirm 2QRU is an esterase if we run the esterase assay using p-nitrophenyl butyrate. | After coming to the conclusion that 2QRU is an esterase, our next research activity would be to test if 2QRU is also a lipase. Three students from The Pingry School in New Jersey performed a lipase assay with 2QRU using nitrophenyl palmitate rather than p-nitrophenyl butyrate to determine if 2QRU is a lipase. Nitrophenyl palmitate has (CH2)14 side chain than nitrophenyl butyrate, so the protein has to act on a bigger substrate. If this assay works for us, then would be able to conclude that 2QRU is also a lipase. Another avenue to explore after confirming 2QRU is a lipase is mutagenesis. If we mutated the catalytic triad responsible for the reaction with nitrophenyl palmitate and ran the lipase assay again, we should see no reaction occur in the cuvette. We can use this to confirm that 2QRU is a lipase. The same thing can be done to confirm 2QRU is an esterase if we run the esterase assay using p-nitrophenyl butyrate. | ||
| - | [[Image:Lipase_Reaction.jpg|thumb|left|400px|Figure | + | [[Image:Lipase_Reaction.jpg|thumb|left|400px|Figure 4: Lipase Reaction]] |
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 14:26, 21 May 2018
2QRU
Here is a cartoon image of my protein:
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644