This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
User:Kayque Alves Telles Silva/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
The most conserved amino acids of Sm and LSm proteins, including Hfq, are the aspartic acid 40 and the glycine 34, determining hydrophobic residues present in the Sm1 motif which maintain the highly distorted Sm1 fold. Tyr56 and Tyr63, highly conserved in the Sm2 motif, are fundamental for the interaction between subunits. The glutamine 8 and tyrosine 42 are highly conserved in Hfq proteins due to their role in uracil binding[1]. The highly variable C-terminus of Hfq proteins has no defined structure and its function is not clearly understood yet[10]. | The most conserved amino acids of Sm and LSm proteins, including Hfq, are the aspartic acid 40 and the glycine 34, determining hydrophobic residues present in the Sm1 motif which maintain the highly distorted Sm1 fold. Tyr56 and Tyr63, highly conserved in the Sm2 motif, are fundamental for the interaction between subunits. The glutamine 8 and tyrosine 42 are highly conserved in Hfq proteins due to their role in uracil binding[1]. The highly variable C-terminus of Hfq proteins has no defined structure and its function is not clearly understood yet[10]. | ||
| - | |||
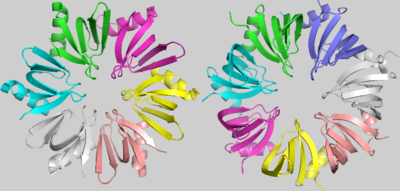
| - | Although the strong conservation of some characteristic amino acids throughout the evolution of LSm proteins, there is an evident and important difference between the bacterial LSm proteins, including Hfq, and the archaeal and eukaryotic LSm protein members. Hfq, as denoted before, is an homo-hexamer, but archaeal and eukaryotic LSm proteins are formed by a homo-heptamer. <scene name='78/789833/Sm_superposition/2'>SM superposition</scene> images shows that this difference results from a shorter Hfq turn between its Sm1 and Sm2 motifs, named the “Variable region”. In addition, in archaeal and eukaryotic LSm proteins, the β3 and β4 strands are extended and the “Variable region” between them forms a little loop within its turn[1]. | ||
| - | |||
| - | |||
[[Image:Hfq_and_SmAP1.png | thumb | right | 400px | Hfq (Left) and SmAP1 (Right)]] | [[Image:Hfq_and_SmAP1.png | thumb | right | 400px | Hfq (Left) and SmAP1 (Right)]] | ||
| + | Although the strong conservation of some characteristic amino acids throughout the evolution of LSm proteins, there is an evident and important difference between the bacterial LSm proteins, including Hfq, and the archaeal and eukaryotic LSm protein members. Hfq, as denoted before, is an homo-hexamer, but archaeal and eukaryotic LSm proteins are formed by a homo-heptamer. <scene name='78/789833/Sm_superposition/2'>SM superposition</scene> images shows that this difference results from a shorter Hfq turn between its Sm1 and Sm2 motifs, named the “Variable region”. In addition, in archaeal and eukaryotic LSm proteins, the β3 and β4 strands are extended and the “Variable region” between them forms a little loop within its turn[1]. | ||
| - | + | The <scene name='78/789833/Subunits_interaction/1'>subunits interaction</scene> is determined by hydrogen bonds between tyrosines present in the β4 and β5 sheets, the Tyr56 and the Tyr63, respectively. The interaction between residues from α1 and the turn between β2 and β3 also contributes to this conformation. The shorter “Variable region” present in Hfq, when compared to the heptameric LSm proteins, allows a closer rotation of the subunits, promoting the hexameric structure of Hfq[1]. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | <scene name='78/789833/Subunits_interaction/1'>subunits interaction</scene> | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | <scene name='78/789833/ | + | The proximal face of Hfq interacts with U-rich 5′-AUUUUUG-3′ RNA sequences, forming a small <scene name='78/789833/Hfq-rna_interaction/2'>Hfq-RNA Interaction</scene> around its pore. Residues such as Tyr42, Lys41 and Gln8 guarantee the bond with uracils and each subunit contacts one nucleotide, except the G residue that remains exposed out of the ring. However, in the predominantly <scene name='78/789833/Rna_distal_face/1'>RNA at distal face</scene>, each subunit bonds with three nucleotides, forming a wider ring of RNA. In this case, A(A/G)N - N being any base - sequences are preferred. |
| - | <scene name='78/789833/ | + | Interestingly, the RNA binding promotes a conformational change in Hfq, highlighted by the pore diameter enlargement. The native protein shows a pore of roughly <scene name='78/789833/Hfq_pore_small/1'>RNA-free Hfq pore size</scene>, but in case of active chaperone activity, the RNA bond promotes a pore diameter deformation to <scene name='78/789833/Hfq_pore_large/1'>RNA-bound Hfq pore size</scene>. |
== Structural highlights == | == Structural highlights == | ||
Revision as of 03:55, 18 June 2018
Hfq
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644