User:Kayque Alves Telles Silva/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 13: | Line 13: | ||
[[Image:Hfq_and_SmAP1.png | thumb | right | 400px | Hfq (Left) and SmAP1 (Right)]] | [[Image:Hfq_and_SmAP1.png | thumb | right | 400px | Hfq (Left) and SmAP1 (Right)]] | ||
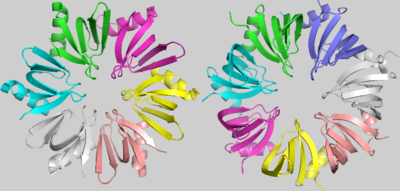
| - | Although some characteristic amino acids were strongly conserved throughout the evolution of LSm proteins, there is an evident and important difference between the bacterial LSm proteins, including Hfq, and the archaeal and eukaryotic LSm protein members. Hfq, as denoted before, is an homo-hexamer, but archaeal and eukaryotic LSm proteins are formed by a homo-heptamer(the image with Hfq and SmAP1, an archaean LSm protein, showcases this difference). <scene name='78/789833/Superposition/ | + | Although some characteristic amino acids were strongly conserved throughout the evolution of LSm proteins, there is an evident and important difference between the bacterial LSm proteins, including Hfq, and the archaeal and eukaryotic LSm protein members. Hfq, as denoted before, is an homo-hexamer, but archaeal and eukaryotic LSm proteins are formed by a homo-heptamer(the image with Hfq and SmAP1, an archaean LSm protein, showcases this difference). <scene name='78/789833/Superposition/4'>Superposition</scene> images shows that this difference results from a shorter Hfq turn between its Sm1 and Sm2 motifs, named the “Variable region”. In addition, in archaeal and eukaryotic LSm proteins, the β3 and β4 strands are extended and the “Variable region” between them forms a little loop within its turn[1]. |
The <scene name='78/789833/Subunits_interaction/1'>subunits interaction</scene> is determined by hydrogen bonds between tyrosines present in the β4 and β5 sheets, the Tyr56 and the Tyr63, respectively. The interaction between residues from α1 and the turn between β2 and β3 also contributes to this conformation. The shorter “Variable region” present in Hfq, when compared to the heptameric LSm proteins, allows a closer rotation of the subunits, promoting the hexameric structure of Hfq[1]. | The <scene name='78/789833/Subunits_interaction/1'>subunits interaction</scene> is determined by hydrogen bonds between tyrosines present in the β4 and β5 sheets, the Tyr56 and the Tyr63, respectively. The interaction between residues from α1 and the turn between β2 and β3 also contributes to this conformation. The shorter “Variable region” present in Hfq, when compared to the heptameric LSm proteins, allows a closer rotation of the subunits, promoting the hexameric structure of Hfq[1]. | ||
Revision as of 04:48, 18 June 2018
Hfq
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644