User:Kayque Alves Telles Silva/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
== Structure == | == Structure == | ||
| - | The <scene name='78/789833/Subunit/1'>basic subunit</scene> which forms the homo-hexamer of Hfq has 77 residues<ref>DOI 10.1093/emboj/cdf322</ref> comprising one N-terminal α helix (α1), five beta sheets (β1-β5) and one variable region. An unstructured carboxy-terminal region, which may have >100 residues in certain species is also present[ | + | The <scene name='78/789833/Subunit/1'>basic subunit</scene> which forms the homo-hexamer of Hfq has 77 residues<ref>DOI 10.1093/emboj/cdf322</ref> comprising one N-terminal α helix (α1), five beta sheets (β1-β5) and one variable region. An unstructured carboxy-terminal region, which may have >100 residues in certain species is also present[5]. The fold of the beta sheets is characterized by two important <scene name='78/789833/Motifs/5'>motifs</scene>, Sm1 and Sm2, used to clusterize the LSm protein family components considering their highlighted conservation throughout Life’s domains. |
| - | The most conserved amino acids of Hfq are the aspartic acid 40 and the glycine 34, determining hydrophobic residues present in the Sm1 motif which maintain the highly distorted Sm1 fold. Tyr56 and Tyr63, highly conserved in the Sm2 motif, are fundamental for the interaction between subunits. The glutamine 8 and tyrosine 42 are highly conserved in Hfq proteins due to their role in uracil binding | + | The most conserved amino acids of Hfq are the aspartic acid 40 and the glycine 34, determining hydrophobic residues present in the Sm1 motif which maintain the highly distorted Sm1 fold. Tyr56 and Tyr63, highly conserved in the Sm2 motif, are fundamental for the interaction between subunits. The glutamine 8 and tyrosine 42 are highly conserved in Hfq proteins due to their role in uracil binding[1]. The highly variable C-terminus of Hfq proteins has no defined structure and its function is not clearly understood yet[5]. |
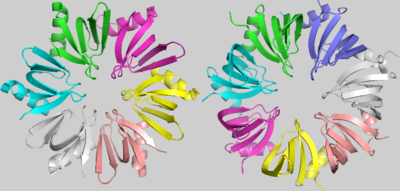
[[Image:Hfq_and_SmAP1.png | thumb | right | 400px | Hfq (Left) and SmAP1 (Right)]] | [[Image:Hfq_and_SmAP1.png | thumb | right | 400px | Hfq (Left) and SmAP1 (Right)]] | ||
| - | Although some characteristic amino acids were strongly conserved throughout the evolution of LSm proteins, there is an evident and important difference between the bacterial LSm proteins, including Hfq, and the archaeal and eukaryotic LSm protein members. Hfq, as denoted before, is an homo-hexamer, but archaeal and eukaryotic LSm proteins are formed by a homo-heptamer(the image with Hfq and SmAP1, an archaean LSm protein, showcases this difference). <scene name='78/789833/Superposition/4'>Superposition</scene> images shows that this difference results from a shorter Hfq turn between its Sm1 and Sm2 motifs, named the “Variable region”. In addition, in archaeal and eukaryotic LSm proteins, the β3 and β4 strands are extended and the “Variable region” between them forms a little loop within its turn | + | Although some characteristic amino acids were strongly conserved throughout the evolution of LSm proteins, there is an evident and important difference between the bacterial LSm proteins, including Hfq, and the archaeal and eukaryotic LSm protein members. Hfq, as denoted before, is an homo-hexamer, but archaeal and eukaryotic LSm proteins are formed by a homo-heptamer(the image with Hfq and SmAP1, an archaean LSm protein, showcases this difference). <scene name='78/789833/Superposition/4'>Superposition</scene> images shows that this difference results from a shorter Hfq turn between its Sm1 and Sm2 motifs, named the “Variable region”. In addition, in archaeal and eukaryotic LSm proteins, the β3 and β4 strands are extended and the “Variable region” between them forms a little loop within its turn[1]. |
| - | The <scene name='78/789833/Subunits_interaction/1'>subunits interaction</scene> is determined by hydrogen bonds between tyrosines present in the β4 and β5 sheets, the '''Tyr56''' and the '''Tyr63''', respectively. The interaction between residues from α1 and the turn between β2 and β3 also contributes to this conformation. The shorter “Variable region” present in Hfq, when compared to the heptameric LSm proteins, allows a closer rotation of the subunits, promoting the hexameric structure of Hfq | + | The <scene name='78/789833/Subunits_interaction/1'>subunits interaction</scene> is determined by hydrogen bonds between tyrosines present in the β4 and β5 sheets, the '''Tyr56''' and the '''Tyr63''', respectively. The interaction between residues from α1 and the turn between β2 and β3 also contributes to this conformation. The shorter “Variable region” present in Hfq, when compared to the heptameric LSm proteins, allows a closer rotation of the subunits, promoting the hexameric structure of Hfq[1]. |
== RNA Interaction== | == RNA Interaction== | ||
| - | The proximal face of Hfq interacts with U-rich 5′-AUUUUUG-3′ RNA sequences, forming a small <scene name='78/789833/Hfq-rna/2'>ring of RNA</scene> around its pore. Residues such as '''Tyr42''', '''Lys41''' and '''Gln8''' guarantee the bond with uracils and each subunit contacts one nucleotide, except the RNA guanine that remains exposed out of the ring. However, in the predominantly <scene name='78/789833/Rna_distal_face/2'>hydrophobic distal face</scene>, each subunit bonds with three nucleotides, forming a wider ring of RNA. In this case, A(A/G)N - N being any base - sequences are preferred | + | The proximal face of Hfq interacts with U-rich 5′-AUUUUUG-3′ RNA sequences, forming a small <scene name='78/789833/Hfq-rna/2'>ring of RNA</scene> around its pore. Residues such as '''Tyr42''', '''Lys41''' and '''Gln8''' guarantee the bond with uracils and each subunit contacts one nucleotide, except the RNA guanine that remains exposed out of the ring. However, in the predominantly <scene name='78/789833/Rna_distal_face/2'>hydrophobic distal face</scene>, each subunit bonds with three nucleotides, forming a wider ring of RNA. In this case, A(A/G)N - N being any base - sequences are preferred[1]. |
| - | Interestingly, the RNA binding promotes a conformational change in Hfq, highlighted by the pore diameter enlargement. The native protein shows a pore of roughly <scene name='78/789833/Hfq_pore_small/2'>12Å</scene>, but in case of active chaperone activity, the RNA bond promotes a pore diameter deformation to <scene name='78/789833/Hfq_pore_large/3'>15Å</scene> | + | Interestingly, the RNA binding promotes a conformational change in Hfq, highlighted by the pore diameter enlargement. The native protein shows a pore of roughly <scene name='78/789833/Hfq_pore_small/2'>12Å</scene>, but in case of active chaperone activity, the RNA bond promotes a pore diameter deformation to <scene name='78/789833/Hfq_pore_large/3'>15Å</scene>[1]. |
== Structural highlights == | == Structural highlights == | ||
| Line 31: | Line 31: | ||
== References == | == References == | ||
<references/> | <references/> | ||
| + | [8]Kajitani, M., Kato, A., Wada, A., Inokuchi, Y., and Ishihama, A. 1994. Regulation of the Escherichia coli hfq gene encoding the host factor for phage Qβ. J. Bacteriol. 176: 531–534. | ||
Revision as of 05:23, 18 June 2018
Hfq
| |||||||||||
References
- ↑ Schumacher MA, Pearson RF, Moller T, Valentin-Hansen P, Brennan RG. Structures of the pleiotropic translational regulator Hfq and an Hfq-RNA complex: a bacterial Sm-like protein. EMBO J. 2002 Jul 1;21(13):3546-56. PMID:12093755 doi:10.1093/emboj/cdf322
- ↑ doi: https://dx.doi.org/10.1046/j.1365-2443.2000.00350.x
- ↑ Wassarman KM, Repoila F, Rosenow C, Storz G, Gottesman S. Identification of novel small RNAs using comparative genomics and microarrays. Genes Dev. 2001 Jul 1;15(13):1637-51. doi: 10.1101/gad.901001. PMID:11445539 doi:http://dx.doi.org/10.1101/gad.901001
- ↑ Updegrove TB, Zhang A, Storz G. Hfq: the flexible RNA matchmaker. Curr Opin Microbiol. 2016 Apr;30:133-138. doi: 10.1016/j.mib.2016.02.003. Epub, 2016 Feb 22. PMID:26907610 doi:http://dx.doi.org/10.1016/j.mib.2016.02.003
- ↑ Santiago-Frangos A, Woodson SA. Hfq chaperone brings speed dating to bacterial sRNA. Wiley Interdiscip Rev RNA. 2018 Jul;9(4):e1475. doi: 10.1002/wrna.1475. Epub 2018, Apr 6. PMID:29633565 doi:http://dx.doi.org/10.1002/wrna.1475
- ↑ Andrade JM, Dos Santos RF, Chelysheva I, Ignatova Z, Arraiano CM. The RNA-binding protein Hfq is important for ribosome biogenesis and affects translation fidelity. EMBO J. 2018 Jun 1;37(11). pii: embj.201797631. doi: 10.15252/embj.201797631., Epub 2018 Apr 18. PMID:29669858 doi:http://dx.doi.org/10.15252/embj.201797631
- ↑ Schumacher MA, Pearson RF, Moller T, Valentin-Hansen P, Brennan RG. Structures of the pleiotropic translational regulator Hfq and an Hfq-RNA complex: a bacterial Sm-like protein. EMBO J. 2002 Jul 1;21(13):3546-56. PMID:12093755 doi:10.1093/emboj/cdf322
- ↑ Vogel J, Luisi BF. Hfq and its constellation of RNA. Nat Rev Microbiol. 2011 Aug 15;9(8):578-89. doi: 10.1038/nrmicro2615. PMID:21760622 doi:http://dx.doi.org/10.1038/nrmicro2615
- ↑ Schumacher MA, Pearson RF, Moller T, Valentin-Hansen P, Brennan RG. Structures of the pleiotropic translational regulator Hfq and an Hfq-RNA complex: a bacterial Sm-like protein. EMBO J. 2002 Jul 1;21(13):3546-56. PMID:12093755 doi:10.1093/emboj/cdf322
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
[8]Kajitani, M., Kato, A., Wada, A., Inokuchi, Y., and Ishihama, A. 1994. Regulation of the Escherichia coli hfq gene encoding the host factor for phage Qβ. J. Bacteriol. 176: 531–534.