We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Frataxin
From Proteopedia
(Difference between revisions)
| Line 56: | Line 56: | ||
In the structure of the channel, the side chains of the hydrophobic aminoacids Leu 145, Val 150 and Leu 152 are exposed to the solvent, creating a <scene name='78/786054/Hydrophobic_lid/3'>hydrophobic lid</scene> around the channel. This hydrophobic lid isolates one of the sides of the channel core, as well as providing a hydrophobic contact surface by which other proteins can interact, hiding their hydrophobic residues from the solvent-rich environment. | In the structure of the channel, the side chains of the hydrophobic aminoacids Leu 145, Val 150 and Leu 152 are exposed to the solvent, creating a <scene name='78/786054/Hydrophobic_lid/3'>hydrophobic lid</scene> around the channel. This hydrophobic lid isolates one of the sides of the channel core, as well as providing a hydrophobic contact surface by which other proteins can interact, hiding their hydrophobic residues from the solvent-rich environment. | ||
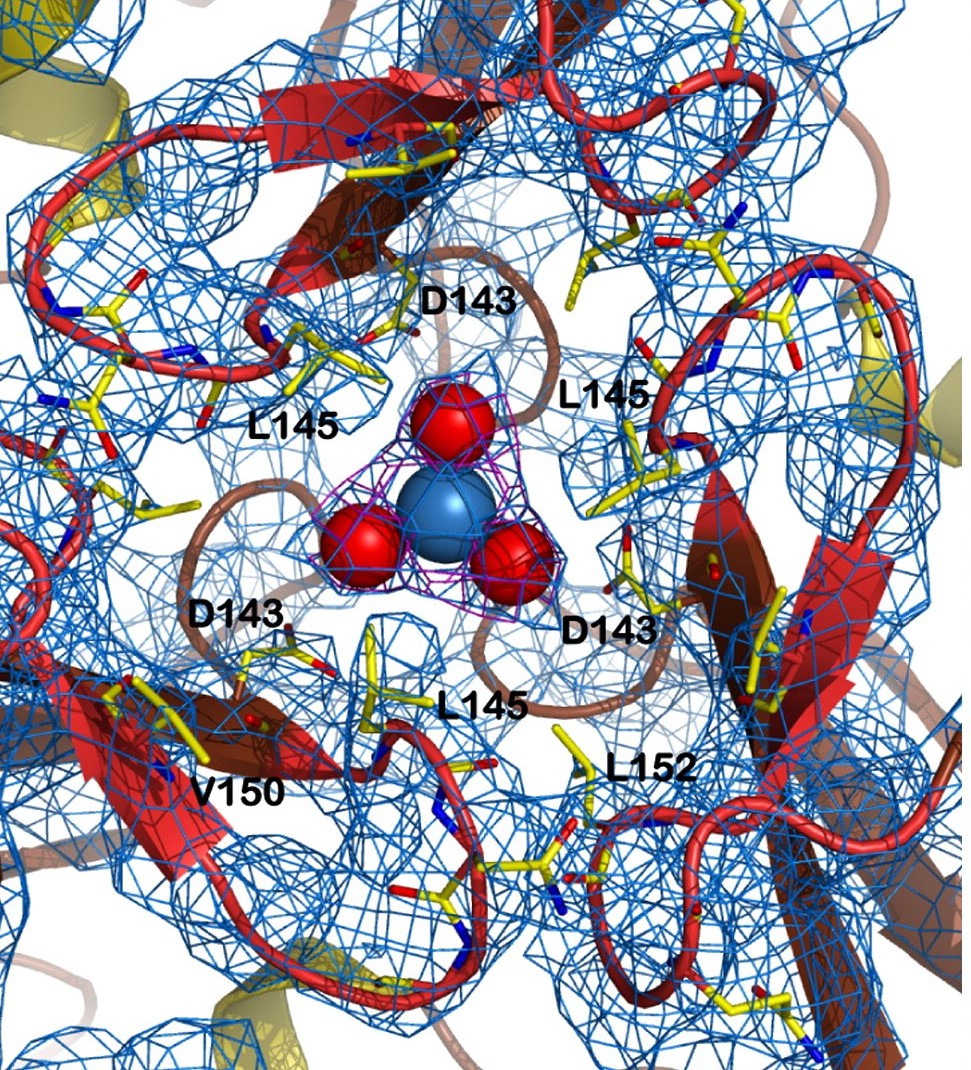
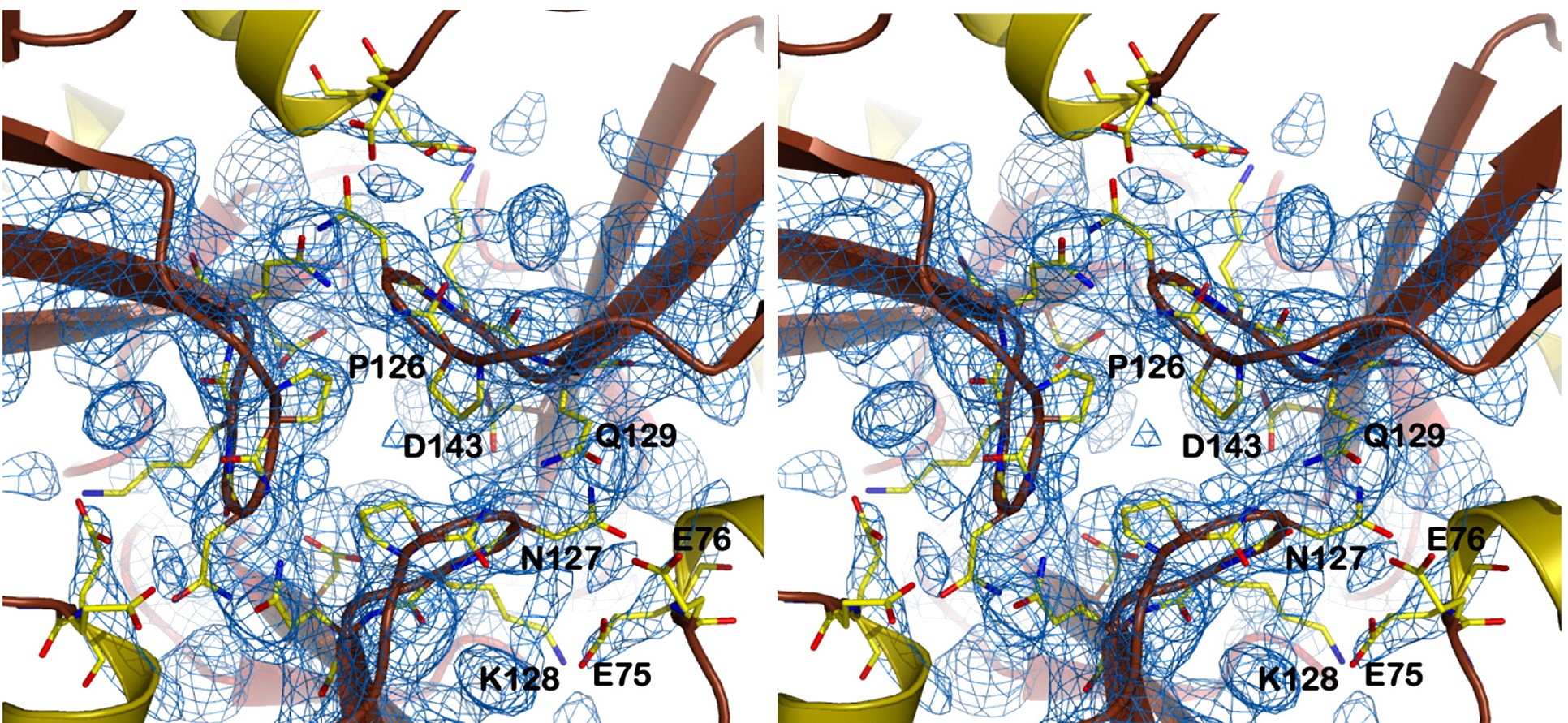
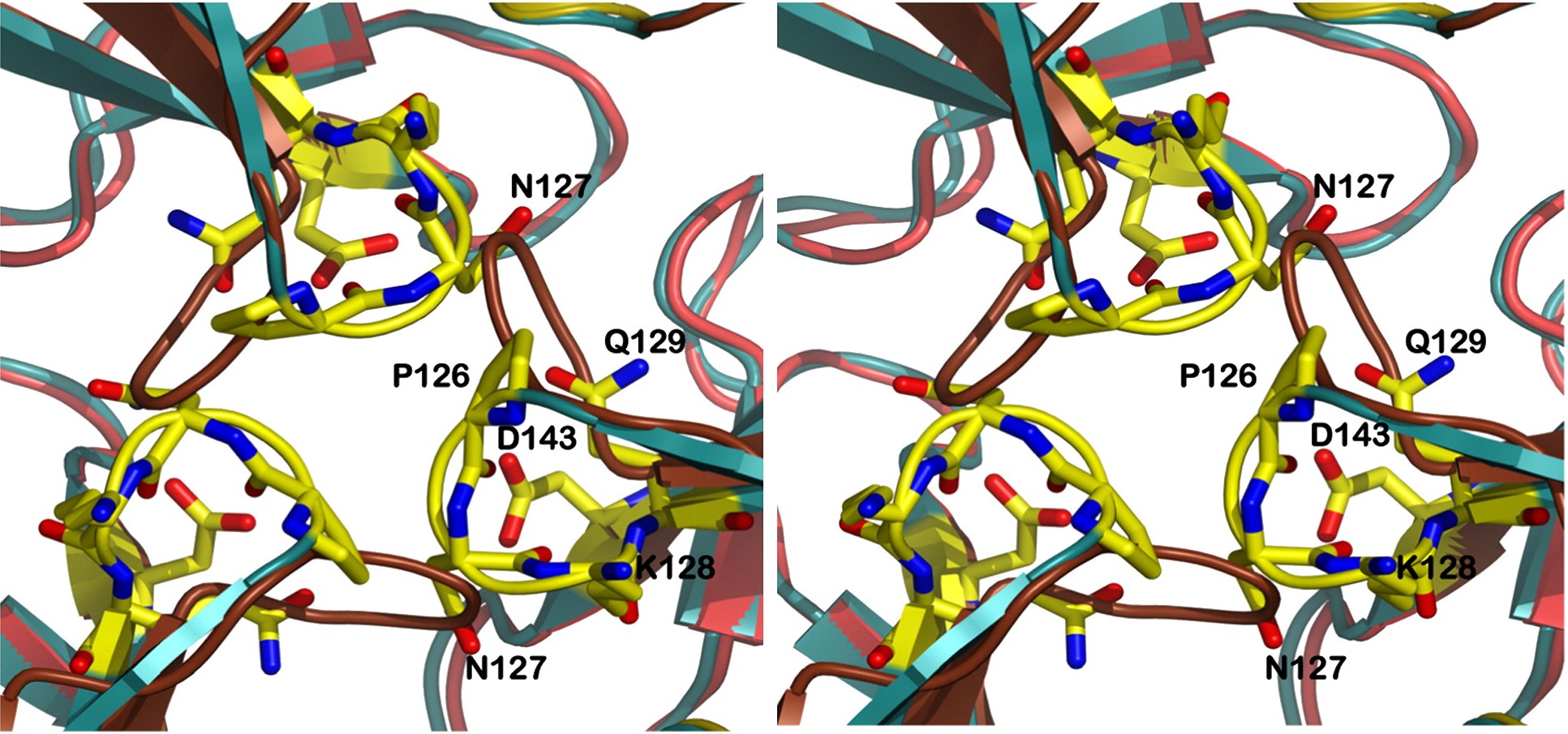
| - | Other structure in the central channel of high importance is the loop formed by <scene name='78/786054/125-128/3'>125-128</scene>. This loop, estabilized by the hidrogen bonds between <scene name='78/786054/143-129__hydrogen_bond/1'>Asp 143-Gln 129</scene>, <scene name='78/786054/Bound_127-75/1'>127-75</scene> and <scene name='79/790348/129-126/1'>129-126</scene>, is present in 2 different conformations. The one just described is involved in further stabilizing the Fe atom | + | Other structure in the central channel of high importance is the loop formed by <scene name='78/786054/125-128/3'>125-128</scene>. This loop, estabilized by the hidrogen bonds between <scene name='78/786054/143-129__hydrogen_bond/1'>Asp 143-Gln 129</scene>, <scene name='78/786054/Bound_127-75/1'>127-75</scene> and <scene name='79/790348/129-126/1'>129-126</scene>, is present in 2 different conformations. The one just described is involved in further stabilizing the Fe atom within the channel. The metal ion binds at around 4 Å from the side chains of the <scene name='78/788815/Iron_channel/1'>three Asp 143 residues</scene> (distances between residues are shown for reference). Laboraroty data from X-ray cristallography suggests the Fe 2+ ion being associated with solvent molecules. In this conformation the PRO126 in pointing toward the center of the channel, further reducing contact of the iron with the enviroment. This create an <scene name='78/786054/Fe_isolation_chamber/1'>isolation chamber</scene> for the iron inside the channel, increasing the stability for the transportation of iron. |
[[Image:carregado fe.jpg]] | [[Image:carregado fe.jpg]] | ||
[[Image:conf_1.jpg]] | [[Image:conf_1.jpg]] | ||
| - | + | the other conformation that the 125-128 loop can take is were it fold outside of the channel, exibiting interactions with the residues N127-Q129-D143. in this conformations, the hydrogens interactions between N127-E75 and Q129-P126 are not present. | |
| + | [[Image:conf,_2.jpg]] | ||
| + | |||
| + | [[Image:lado_1.jpg]] | ||
| - | Within the channel, the metal ion binds at around 4 Å from the side chains of the <scene name='78/788815/Iron_channel/1'>three Asp 143 residues</scene> (distances between residues are shown for reference). Laboraroty data from X-ray cristallography suggests the Fe 2+ ion being associated with solvent molecules. | ||
| Line 78: | Line 80: | ||
<scene name='78/786054/143-129-126__hydrogen_bond/1'>143-129-126 hydrogen bond</scene> | <scene name='78/786054/143-129-126__hydrogen_bond/1'>143-129-126 hydrogen bond</scene> | ||
| - | + | ||
Revision as of 06:08, 18 June 2018
Frataxin
| |||||||||||
References
Proteopedia Page Contributors and Editors (what is this?)
João Victor Paccini Coutinho, Michal Harel, Rebeca B. Candia