Kisker lab: 5B5Q
From Proteopedia
(→Chlamydia trachomatis inhibits apoptosis) |
(→Structure) |
||
| Line 34: | Line 34: | ||
<scene name='78/781027/Paneld/3'>Panel D:</scene> The active site residues Cys 345, His, and Asp form the catalytic triad. Instead of showing omit density like in the paper, we are showing 2Fo-Fc <jmol><jmollink><text>density</text><script>isosurface s_one color silver "http://proteopedia.org/wiki/images/6/61/Map.jvxl" mesh</script></jmollink></jmol>. Center on <jmol><jmollink><text>His 275</text><script>zoom *3; select 275 and sidechain; center selected; background black</script></jmollink></jmol>. | <scene name='78/781027/Paneld/3'>Panel D:</scene> The active site residues Cys 345, His, and Asp form the catalytic triad. Instead of showing omit density like in the paper, we are showing 2Fo-Fc <jmol><jmollink><text>density</text><script>isosurface s_one color silver "http://proteopedia.org/wiki/images/6/61/Map.jvxl" mesh</script></jmollink></jmol>. Center on <jmol><jmollink><text>His 275</text><script>zoom *3; select 275 and sidechain; center selected; background black</script></jmollink></jmol>. | ||
| - | |||
| - | ===Substrate binding site=== | ||
| - | |||
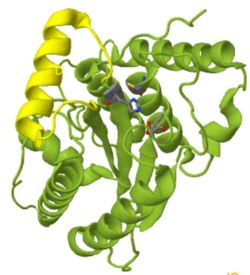
| - | <scene name='78/781027/Bonus/5'>Bonus figure:</scene> The active-site cysteine sidechain acting as a nucleophile in the hydrolysis reaction is buried surprisingly deeply, barely visible in the surface view (yellow). The alpha helix inserted between strand 1 and two (shown in yellow) is above the substrate binding cavity, with Val 271 blocking access to the active site. | ||
===Superposition with product complex of SENP8=== | ===Superposition with product complex of SENP8=== | ||
| Line 63: | Line 59: | ||
| + | ===Substrate binding site=== | ||
| + | |||
| + | <scene name='78/781027/Bonus/5'>Bonus figure:</scene> The active-site cysteine sidechain acting as a nucleophile in the hydrolysis reaction is buried surprisingly deeply, barely visible in the surface view (yellow). The alpha helix inserted between strand 1 and two (shown in yellow) is above the substrate binding cavity, with Val 271 blocking access to the active site. | ||
</StructureSection> | </StructureSection> | ||
Revision as of 14:37, 26 June 2018
Contents |
How this page was created
The goal of this page is to provide three-dimensional and interactive figures to explore the structure of a protein involved in bacterial infections (PDB code 5B5Q). The starting point are the figures found in the paper describing the 5B5Q structure. Biochemistry students from Westfield State University recreated these figures as best as possible in jmol, and revised them after getting feedback from researchers who authored the primary citation (Kisker lab in Würzburg, Germany). A special thank you goes to Ose Aimua, Nina Aldabayeva, Faiqa Ashraf, Kaleigh Florek, Ellie Hoeg, Aya Maytham, Christian Mikule, Brigid Murray, Kevin Pelletier, Brandon Reder, Erin Riley, Brian Schuler, and Jakob Wyman for the revisions of figures and for working on the links to other proteopedia pages.
If you are interested to see the jmol scripts used to make these figures, take a look at the discussion page (2nd tab above).
Chlamydia trachomatis inhibits apoptosis
Chlamydia trachomatis is a bacterium that reproduces inside human cells. One defense of the human body against Chlamydia is to kill affected human cells before Chlamydia reproduces and infects more human cells. This is done through a process called apoptosis, programmed cell death. One player in apoptosis is the human protein Mcl-1. High Mcl-1 levels inhibit one of the signalling pathways that lead to apoptosis. Chlamydia inhibits Mcl-1 degradation so that Mcl-1 levels remain high.
Protein ubiquitination and degradation
Human cells have a protein assembly called the proteasome, which specializes in degrading proteins. Ubiquitin is a small, highly soluble protein; when ubiquitin chains are attached to other proteins in a certain way, it acts as a signal for protein degradation. The proteasome only degrades proteins that are poly-ubiquitinated, i.e. are covalently linked to a linear chain of ubiquitins. The covalent link is between the amino group of a lysine side chain and the carboxylic acid of a glycine at the C-terminus of ubiquitin.
The deubiquitinase activity of Cdu1 stabilizes Mcl-1
The Chlamydia protein Cdu1 is a protease that catalyzes the hydrolysis of ubiquitin chains from Mcl-1. When polyubiquitinated, Mcl-1 is destined to be degraded by the proteasome, lowering the level of Mcl-1 and subsequently leading to apoptosis. The activity of Cdu1 counteracts this by removing the ubiquitin, thus leading to higher levels of Mcl-1 in the cell.
Structure
| |||||||||||
Relevant Links
5b5q : autogenerated Proteopedia page on coordinates
Ubiquitin Structure & Function : Proteopedia article on Ubiquitin Structure, function and conjugation
Ubiquitin chains : Proteopedia article on Ubiquitin Chains
Category: Kisker C : list of structures associated with the Kisker lab