Penicillin acylase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
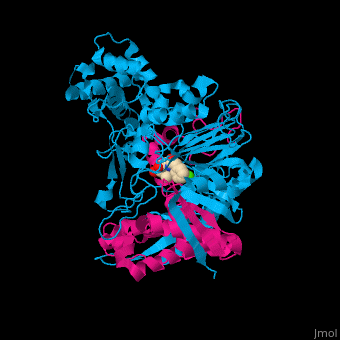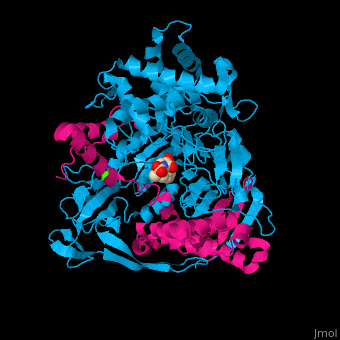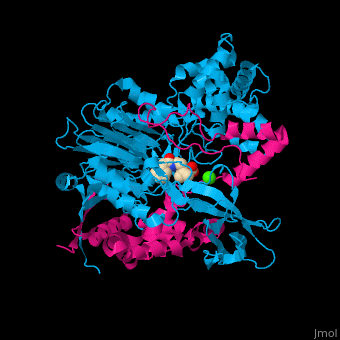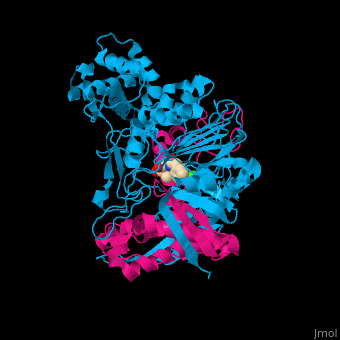
| - | <StructureSection load='1fxv' size=' | + | <StructureSection load='1fxv' size='350' side='right' scene='52/525137/Cv/1' caption='E. coli penicillin acylase small α subunit (magenta) and large β subunit (deepskyblue) complex with penicillin and Ca+2 ion (green), [[1fxv]]' pspeed='8'> |
== Function == | == Function == | ||
'''Penicillin acylase''' (PAH) catalyzes the conversion of penicillin to 6-amino-penicillanate and phenylacetate (PA). PAH contains 2 non-identical subunits. The larger β subunit contains a phenylmethylsulfonyl residue which is required for enzymatic activity. PAH participates in penicillin and cephalosporin biosynthesis<ref>PMID:7816145</ref>. | '''Penicillin acylase''' (PAH) catalyzes the conversion of penicillin to 6-amino-penicillanate and phenylacetate (PA). PAH contains 2 non-identical subunits. The larger β subunit contains a phenylmethylsulfonyl residue which is required for enzymatic activity. PAH participates in penicillin and cephalosporin biosynthesis<ref>PMID:7816145</ref>. | ||
Revision as of 08:01, 16 August 2018
| |||||||||||
3D structures of penicillin acylase
Updated on 16-August-2018
References
- ↑ Duggleby HJ, Tolley SP, Hill CP, Dodson EJ, Dodson G, Moody PC. Penicillin acylase has a single-amino-acid catalytic centre. Nature. 1995 Jan 19;373(6511):264-8. PMID:7816145 doi:http://dx.doi.org/10.1038/373264a0
- ↑ Volpato G, Rodrigues RC, Fernandez-Lafuente R. Use of enzymes in the production of semi-synthetic penicillins and cephalosporins: drawbacks and perspectives. Curr Med Chem. 2010;17(32):3855-73. PMID:20858215
- ↑ Alkema WB, Hensgens CM, Kroezinga EH, de Vries E, Floris R, van der Laan JM, Dijkstra BW, Janssen DB. Characterization of the beta-lactam binding site of penicillin acylase of Escherichia coli by structural and site-directed mutagenesis studies. Protein Eng. 2000 Dec;13(12):857-63. PMID:11239085