We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Telomerase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
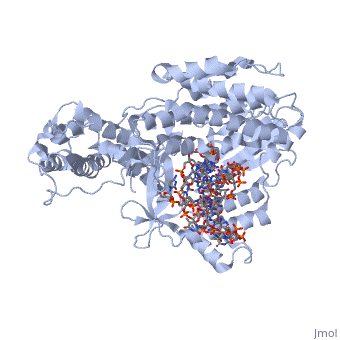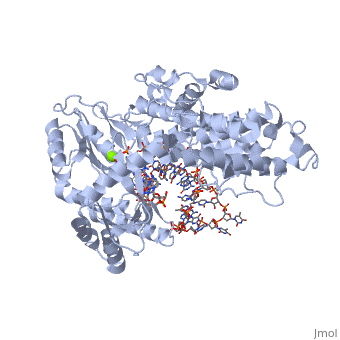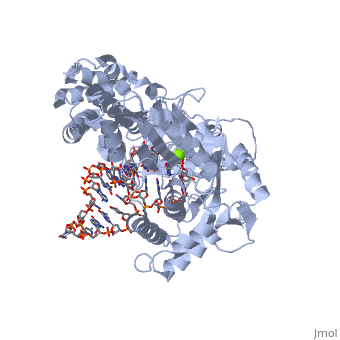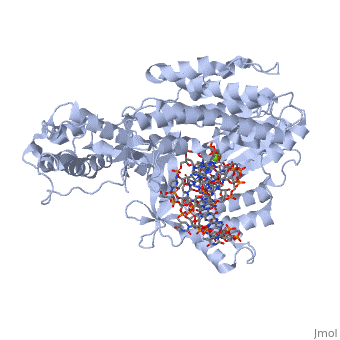
<StructureSection load='3kyl' size='350' side='right' caption='Telomerase: bound to telomeric DNA complex with Ca+2 ion (green) [[3kyl]]' scene=''> | <StructureSection load='3kyl' size='350' side='right' caption='Telomerase: bound to telomeric DNA complex with Ca+2 ion (green) [[3kyl]]' scene=''> | ||
== Introduction == | == Introduction == | ||
| - | The '''telomerase''' is a complex composed of a | + | The '''telomerase''' is a complex composed of a '''Telomerase RNA''' primer and protein '''telomerase reverse transcriptase''' that adds G-rich repeated DNA sequences to a 3' DNA strand in eukaryotic cells <ref name='complex'>DOI: 10.1146/annurev.bi.61.070192.000553</ref>. This ribonucleoprotein acts as a reverse transcriptase that creates a DNA sequence complementary to its RNA primer in its catalytic subunit, TERT <ref name='end'>DOI: 10.1002/anie.201002387</ref>. The sequences attached to the end of DNA is non-coding DNA that functions as protection from mutation and degradation. As DNA replications occurs, the polymerase that copies in the 5' to 3' direction, cannot copy the end of the lagging strand which leaves unpaired bases that may code for a specific gene <ref name='corey'>DOI 10.1016/j.chembiol.2009.12.001</ref>. Multiple unpaired ends can form hydrogen bonds or even swap genetic material. The telomerase fixes this issue and lengthens the strands, because the replication process slowly shortens the ends of chromosomes. This enzyme also plays a role in coordinating cell division. Complementary issues of aging and cancer describe the inactivation or over-activation of telomerase activity <ref name='corey'/>. |
== History == | == History == | ||
| Line 32: | Line 32: | ||
{{#tree:id=OrganizedByTopic|openlevels=0| | {{#tree:id=OrganizedByTopic|openlevels=0| | ||
| + | *Telomerase reverse transcriptase | ||
| + | |||
| + | **[[2b2a]] - TtTERT telomerase catalytic subunit (mutant) - ''Tetrahymena thermophila''<br /> | ||
| + | **[[2r4g]] - TtTERT telomerase catalytic subunit<br /> | ||
| + | **[[6d6v]] – TtTERT + telomerase-associated protein + TEB2 + RNA + DNA – Cryo EM<br /> | ||
| + | **[[5c9h]] - TtTERT catalytic subunit + DNA<br /> | ||
| + | **[[3du6]], [[3du5]] - TcTERT telomerase catalytic subunit - ''Tribolium castaneum''<br /> | ||
| + | **[[3kyl]] - TcTERT telomerase catalytic subunit + DNA<br /> | ||
| + | **[[4o26]] – TERT RNA-binding domain + RNA – rice fish<br /> | ||
| + | **[[4lmo]] - TERT telomerase RNA-binding domain – tiger puffer<br /> | ||
| + | **[[5lgf]] - TERT N-terminal - ''Ogataea polymorpha'' - NMR<br /> | ||
| + | **[[5ugw]] - hTERT thumb domain<br /> | ||
| + | **[[5cqg]] - TERT + inhibitor - red flour beetle<br /> | ||
| + | **[[5npt]] – TERT N-terminal – ''Ogataea polymorpha''<br /> | ||
*Telomerase RNA | *Telomerase RNA | ||
Revision as of 21:15, 24 September 2018
| |||||||||||
Telomerase 3D structures
Updated on 24-September-2018
References
- ↑ 1.0 1.1 Blackburn EH. Telomerases. Annu Rev Biochem. 1992;61:113-29. PMID:1497307 doi:http://dx.doi.org/10.1146/annurev.bi.61.070192.000553
- ↑ 2.0 2.1 Blackburn EH. Telomeres and telomerase: the means to the end (Nobel lecture). Angew Chem Int Ed Engl. 2010 Oct 4;49(41):7405-21. doi: 10.1002/anie.201002387. PMID:20821774 doi:http://dx.doi.org/10.1002/anie.201002387
- ↑ 3.0 3.1 3.2 3.3 3.4 Corey DR. Telomeres and telomerase: from discovery to clinical trials. Chem Biol. 2009 Dec 24;16(12):1219-23. doi: 10.1016/j.chembiol.2009.12.001. PMID:20064431 doi:http://dx.doi.org/10.1016/j.chembiol.2009.12.001
- ↑ 4.0 4.1 4.2 Gillis AJ, Schuller AP, Skordalakes E. Structure of the Tribolium castaneum telomerase catalytic subunit TERT. Nature. 2008 Oct 2;455(7213):633-7. Epub 2008 Aug 31. PMID:18758444 doi:http://dx.doi.org/10.1038/nature07283
- ↑ 5.0 5.1 Mitchell M, Gillis A, Futahashi M, Fujiwara H, Skordalakes E. Structural basis for telomerase catalytic subunit TERT binding to RNA template and telomeric DNA. Nat Struct Mol Biol. 2010 Apr;17(4):513-8. Epub 2010 Mar 28. PMID:20357774 doi:10.1038/nsmb.1777