Sandbox RDE-1
From Proteopedia
| Line 5: | Line 5: | ||
== Structure == | == Structure == | ||
| - | All "Argonaute"/Piwi proteins possess three primary domains forming a crescent-shaped base: the PAZ, MID, and PIWI domains. The amino-terminal PAZ domain uses its oligonucleotide-binding (OB) fold to secure the 3′ end of the small RNA guide strand to ''Argonaute'' protein. A conserved hydrophobic cavity within the PAZ domain recognizes the characteristic two-nucleotide, 3′ overhanging end of the guide-passenger siRNA generated by Dicer. The MID domain anchors the 5′ monophosphate of a siRNA to the ''Argonaute'' protein, securing the guide through multiple cycles of target cleavage. In vitro, studies suggest that 5′ phosphate binding helps align the small RNA on the surface of "Argonaute" protein, ensuring that the correct bond of the target is positioned in the endonuclease active site.<ref name=four>PMID: 21683893</ref> In other words, the PAZ and MID domains orient and anchor the double-stranded siRNA by binding to the 3’ and 5’ termini, respectively, leaving the internal nucleotides accessible for base pairing.<ref name=four/><ref name=three>PMID:15284453</ref> The carboxy-terminal PIWI domain resembles nuclease RNase H in which it folds into an RNase H-like structure. This domain contains three conserved amino acids, aspartate-aspartate-histidine, that form a catalytic triad "DDH".<ref name=three/> The crystal structure of RDE-1 has not been fully elucidated, but can be assumed to closely resemble its human homologs. The full length of RDE-1 protein is 1020 amino acids (aa)<ref name=two/> in which about 110 of those aa makes up the PAZ domain and 300 aa makes up the PIWI domain.<ref>DOI: 10.1016/s0968-0004(00)01641-8</ref> | + | All "Argonaute"/Piwi proteins possess three primary domains forming a crescent-shaped base: the PAZ, MID, and PIWI domains. The amino-terminal PAZ domain uses its oligonucleotide-binding (OB) fold to secure the 3′ end of the small RNA guide strand to ''Argonaute'' protein. A conserved hydrophobic cavity within the PAZ domain recognizes the characteristic two-nucleotide, 3′ overhanging end of the guide-passenger siRNA generated by Dicer. The MID domain anchors the 5′ monophosphate of a siRNA to the ''Argonaute'' protein, securing the guide through multiple cycles of target cleavage. In vitro, studies suggest that 5′ phosphate binding helps align the small RNA on the surface of "Argonaute" protein, ensuring that the correct bond of the target is positioned in the endonuclease active site.<ref name=four>PMID: 21683893</ref> In other words, the PAZ and MID domains orient and anchor the double-stranded siRNA by binding to the 3’ and 5’ termini, respectively, leaving the internal nucleotides accessible for base pairing.<ref name=four/><ref name=three>PMID:15284453</ref> The carboxy-terminal PIWI domain resembles nuclease RNase H in which it folds into an RNase H-like structure. This domain contains three conserved amino acids, aspartate-aspartate-histidine, that form a catalytic triad "DDH".<ref name=three/> The crystal structure of RDE-1 has not been fully elucidated, but can be assumed to closely resemble its human homologs. The full length of RDE-1 protein is 1020 amino acids (aa)<ref name=two/> in which about 110 of those aa makes up the PAZ domain and 300 aa makes up the PIWI domain.<ref> DOI: 10.1016/s0968-0004(00)01641-8</ref> |
== Importance/Function == | == Importance/Function == | ||
| - | [[Image:RDE-1 Proposed Fxn.png|300px||left|thumb|Proposed RNAi pathway for exogenous trigger dsRNA in C. elegans.<ref>https://en.wikipedia.org/wiki/RDE-1#/media/File:Exogenous_RNAi_Pathway_in_C._elegans,_edited.svg</ref>]] | + | [[Image:RDE-1 Proposed Fxn.png|300px||left|thumb|Proposed RNAi pathway for exogenous trigger dsRNA in C. elegans.<ref>https://en.wikipedia.org/wiki/RDE-1#/media/File:Exogenous_RNAi_Pathway_in_C._elegans,_edited.svg</ref>]] '''RDE-1''' is not required for the initial processing of the trigger RNA into siRNAs in RNAi, but it is required in the effector step. The trigger dsRNA is bound by RDE-4 onto a '''Dicer''' complex and this complex cleaves the dsRNA into 21-25nt primary siRNA. The siRNA binds to RDE-1 promoting the formation of the RNA-induced silencing complex (RISC) and RDE-1 shuttles the siRNA to that effector complex. The RNase H activity in PIWI domain in RDE-1 facilitates siRNA maturation, cleaving siRNA into a single stranded siRNA or guide RNA while RISC is activated when ATP is added to the complex and utilized the guide RNA on RDE-1 to base pair with the target transcript. The activation of RISC promotes the recruitment of RNA-dependent RNA polymerase (RdRP) which triggers the amplification of the secondary siRNAs to exhibit target transcript degradation. |
== Disease == | == Disease == | ||
Revision as of 02:23, 9 October 2018
Contents |
Introduction
|
The rde-1 gene is a member of the Argonaute gene family. Proteins from "Argonaute" family form an evolutionarily conserved family whose members silence gene expression in pathways such as RNA interference (RNAi). Argonaute family proteins can be divided into two types, AGO and Piwi proteins, depending on the small RNA they bonded to. Both types of Argonaute proteins bind 21–35 nucleotide-long small RNA guides whose sequence identifies the genes to be silenced.[1] RDE-1 (RNAi-DEfective 1), a primary Argonaute protein, is required for RNA-mediated interference in Caenorhabditis elegans; thus, it is also known as RNAi promoting factor. Its gene locus was first characterized in C. elegans mutants resistant to RNAi, and was found to be a member of the Piwi gene family that includes plant, Drosophila, and vertebrate homologs.[2]
Structure
All "Argonaute"/Piwi proteins possess three primary domains forming a crescent-shaped base: the PAZ, MID, and PIWI domains. The amino-terminal PAZ domain uses its oligonucleotide-binding (OB) fold to secure the 3′ end of the small RNA guide strand to Argonaute protein. A conserved hydrophobic cavity within the PAZ domain recognizes the characteristic two-nucleotide, 3′ overhanging end of the guide-passenger siRNA generated by Dicer. The MID domain anchors the 5′ monophosphate of a siRNA to the Argonaute protein, securing the guide through multiple cycles of target cleavage. In vitro, studies suggest that 5′ phosphate binding helps align the small RNA on the surface of "Argonaute" protein, ensuring that the correct bond of the target is positioned in the endonuclease active site.[1] In other words, the PAZ and MID domains orient and anchor the double-stranded siRNA by binding to the 3’ and 5’ termini, respectively, leaving the internal nucleotides accessible for base pairing.[1][3] The carboxy-terminal PIWI domain resembles nuclease RNase H in which it folds into an RNase H-like structure. This domain contains three conserved amino acids, aspartate-aspartate-histidine, that form a catalytic triad "DDH".[3] The crystal structure of RDE-1 has not been fully elucidated, but can be assumed to closely resemble its human homologs. The full length of RDE-1 protein is 1020 amino acids (aa)[2] in which about 110 of those aa makes up the PAZ domain and 300 aa makes up the PIWI domain.[4]
Importance/Function
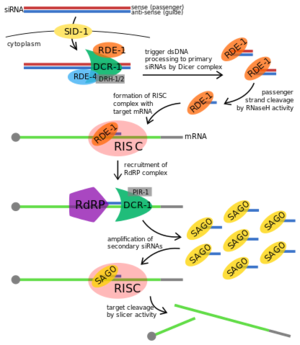
Disease
Relevance
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
</StructureSection>
References
- ↑ 1.0 1.1 1.2 Cenik ES, Zamore PD. Argonaute proteins. Curr Biol. 2011 Jun 21;21(12):R446-9. doi: 10.1016/j.cub.2011.05.020. PMID:21683893 doi:http://dx.doi.org/10.1016/j.cub.2011.05.020
- ↑ 2.0 2.1 Tabara H, Sarkissian M, Kelly WG, Fleenor J, Grishok A, Timmons L, Fire A, Mello CC. The rde-1 gene, RNA interference, and transposon silencing in C. elegans. Cell. 1999 Oct 15;99(2):123-32. PMID:10535731
- ↑ 3.0 3.1 Song JJ, Smith SK, Hannon GJ, Joshua-Tor L. Crystal structure of Argonaute and its implications for RISC slicer activity. Science. 2004 Sep 3;305(5689):1434-7. Epub 2004 Jul 29. PMID:15284453 doi:10.1126/science.1102514
- ↑ Cerutti L, Mian N, Bateman A. Domains in gene silencing and cell differentiation proteins: the novel PAZ domain and redefinition of the Piwi domain. Trends Biochem Sci. 2000 Oct;25(10):481-2. doi: 10.1016/s0968-0004(00)01641-8. PMID:11050429 doi:http://dx.doi.org/10.1016/s0968-0004(00)01641-8
- ↑ https://en.wikipedia.org/wiki/RDE-1#/media/File:Exogenous_RNAi_Pathway_in_C._elegans,_edited.svg
