SN2 reaction
From Proteopedia
(trying to recover the model file) |
|||
| Line 5: | Line 5: | ||
== S<sub>N</sub>2-Substitution of chloride and methanol == | == S<sub>N</sub>2-Substitution of chloride and methanol == | ||
| - | <Structure load='SN2_animation3d. | + | <Structure load='SN2_animation3d.xyz' size='400' frame='true' align='right' caption='' scene='' /> |
Typically, alkanes with a substituent in primary position undergo S<sub>N</sub>2 reactions. In contrast to a S<sub>N</sub>1 reaction, no stable carbo cation can be formed. Therefore, another way will be taken. | Typically, alkanes with a substituent in primary position undergo S<sub>N</sub>2 reactions. In contrast to a S<sub>N</sub>1 reaction, no stable carbo cation can be formed. Therefore, another way will be taken. | ||
Revision as of 14:13, 17 October 2018
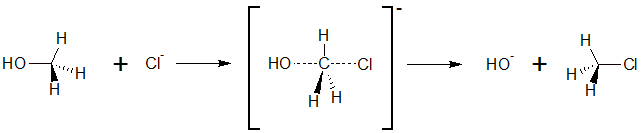
SN2 reaction is a basic reaction type in organic chemistry. The letter SN stand for nulceophilic Substitution, the number 2 stands for bimolecular. This means that both reactions partners are involved in the reaction rate determining step. It also exists an SN1 reaction; here, only one reaction partner is involved in this step.
On the other side, SN2 reactions are characterised for exchanging substituents. The substituent that leaves the molecule is called leaving group.
SN2-Substitution of chloride and methanol
|
Typically, alkanes with a substituent in primary position undergo SN2 reactions. In contrast to a SN1 reaction, no stable carbo cation can be formed. Therefore, another way will be taken. The SN2 by establishing a so called intermediate state. This means that both educts come close to each other. By this, the bond of the leaving group is partly broken, and the bond to the new group is partly formed. The formation of this intermediate state is the rate determining step of the reaction. In the , the bond of the leaving group is completely broken, and at the same time the bond to the new substituent is completely formed.
The SN2 reaction has a very interesting stereochemistry. inversion of the stereocenter...
This demo was adapted from http://www.chemieunterricht-interaktiv.de/en/animations/sn2_substitution/sn2_substitution_3d.html by Dr. V. Pietzner, part of the ChiLe project
Proteopedia Page Contributors and Editors (what is this?)
Joel L. Sussman, Jaime Prilusky, Angel Herraez, Verena Pietzner