Proteopedia:Featured SEL/0
From Proteopedia
(Difference between revisions)
(New page: <table> <tr><td>Image:Oct2017.png</td></tr> <tr><td>'''HIV-1 protease'''</td></tr> <tr><td>''David Canner''</td></tr> <tr><td>The X-ray structure of HIV-1 protease reveals that it is c...) |
|||
| Line 1: | Line 1: | ||
<table> | <table> | ||
| - | <tr><td>[[Image: | + | <tr><td>[[Image:Anim HIV protease.gif]]</td></tr> |
<tr><td>'''HIV-1 protease'''</td></tr> | <tr><td>'''HIV-1 protease'''</td></tr> | ||
<tr><td>''David Canner''</td></tr> | <tr><td>''David Canner''</td></tr> | ||
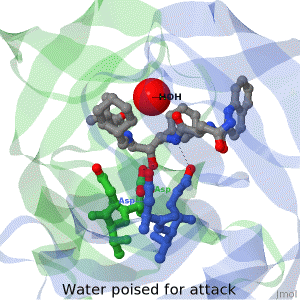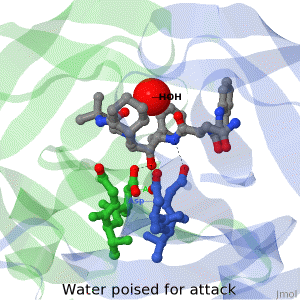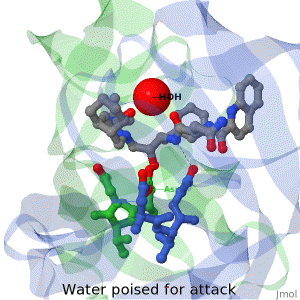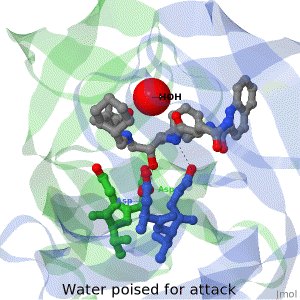
<tr><td>The X-ray structure of HIV-1 protease reveals that it is composed of two symmetrically related subunits, each consisting of 99 amino acid residues. The subunits come together in such as way as to form a tunnel where they meet. This tunnel is of critical importance because the active site of the protease is located in its interior. The active site consists of two Asp-Thr-Gly conserved sequences, making it a member of the aspartyl protease family. The two Asp's are essential catalytic residues either interact with the incoming water OR protonate the carbonyl to make the carbon more electrophilic for the incoming water.</td></tr> | <tr><td>The X-ray structure of HIV-1 protease reveals that it is composed of two symmetrically related subunits, each consisting of 99 amino acid residues. The subunits come together in such as way as to form a tunnel where they meet. This tunnel is of critical importance because the active site of the protease is located in its interior. The active site consists of two Asp-Thr-Gly conserved sequences, making it a member of the aspartyl protease family. The two Asp's are essential catalytic residues either interact with the incoming water OR protonate the carbonyl to make the carbon more electrophilic for the incoming water.</td></tr> | ||
</table> | </table> | ||