Proteopedia:Featured JRN/1
From Proteopedia
(Difference between revisions)
(shorten the content) |
(shorten the content) |
||
| Line 2: | Line 2: | ||
<tr><td>[[Image:Anim Samatey2 FigA I.gif]]</td></tr> | <tr><td>[[Image:Anim Samatey2 FigA I.gif]]</td></tr> | ||
<tr><td><div class="scrolling">'''Structural flexibility of the periplasmic protein, FlgA, regulates flagellar P-ring assembly in ''Salmonella enterica''.'''<br> | <tr><td><div class="scrolling">'''Structural flexibility of the periplasmic protein, FlgA, regulates flagellar P-ring assembly in ''Salmonella enterica''.'''<br> | ||
| - | ''H Matsunami, YH Yoon, VA Meshcheryakov, K Namba, FA Samatey''. ''Scientific Reports'' | + | ''H Matsunami, YH Yoon, VA Meshcheryakov, K Namba, FA Samatey''. ''Scientific Reports'' 2016 doi: [http://dx.doi.org/10.1038/srep27399 10.1038/srep27399]<br> |
A periplasmic flagellar chaperone protein, FlgA, is required for P-ring assembly in bacterial flagella of taxa such as ''Salmonella enterica'' or ''Escherichia coli''. Here we present the open and closed crystal structures of FlgA from ''Salmonella enterica serovar Typhimurium'', grown under different crystallization conditions. An intramolecular disulfide cross-linked form of FlgA caused a dominant negative effect on motility of the wild-type strain. | A periplasmic flagellar chaperone protein, FlgA, is required for P-ring assembly in bacterial flagella of taxa such as ''Salmonella enterica'' or ''Escherichia coli''. Here we present the open and closed crystal structures of FlgA from ''Salmonella enterica serovar Typhimurium'', grown under different crystallization conditions. An intramolecular disulfide cross-linked form of FlgA caused a dominant negative effect on motility of the wild-type strain. | ||
Revision as of 19:04, 18 October 2018
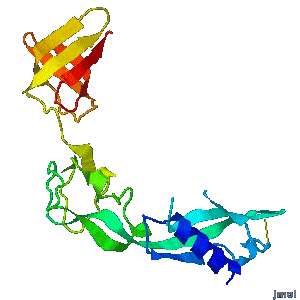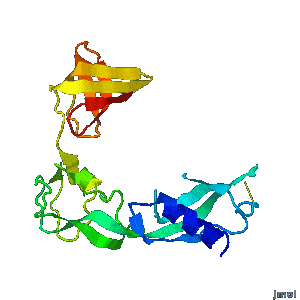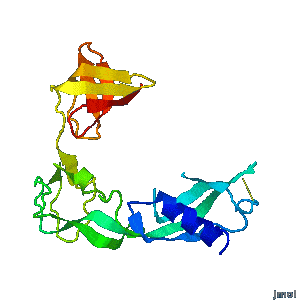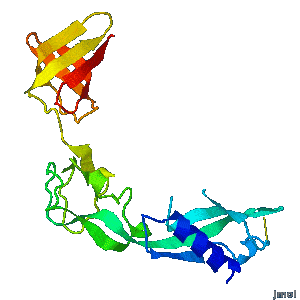 |
Structural flexibility of the periplasmic protein, FlgA, regulates flagellar P-ring assembly in Salmonella enterica.
H Matsunami, YH Yoon, VA Meshcheryakov, K Namba, FA Samatey. Scientific Reports 2016 doi: 10.1038/srep27399 >>> Visit this I3DC complement >>> |
