We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1473
From Proteopedia
(Difference between revisions)
| Line 23: | Line 23: | ||
| - | + | == THE GENUIS OF GPCRs == | |
| - | + | <Structure load='4j4q' size='300' frame='true' align='right' caption='Rhodopsin-like GPCR structure' /> | |
| - | + | ||
| - | <Structure load='4j4q' size='300' frame='true' align='right' caption=' | + | |
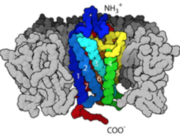
<StructureSection load='2rh1' size='300' side='right' caption='Human Beta adrenergic receptor with bound ligand' scene='80/800652/Showing_ligand/1' > | <StructureSection load='2rh1' size='300' side='right' caption='Human Beta adrenergic receptor with bound ligand' scene='80/800652/Showing_ligand/1' > | ||
| - | + | == NTRODUCTION == | |
Within the course of our daily activities, we experience several situations that cause us to jump on our feet and also, initiate some kind of response to these situations. Our bodies have an outstanding way of preparing us for such events as to whether flee or fight and it does this by making energy in the form of glucose available in the blood to support our reactions to these events. Our response is effectively mediated through reflex actions which are propagated through networks of neurons that are wired all over our body and eventually to the brain. One of the neurotransmitters produced through the coordination of these neuronal networks is epinephrine. Epinephrine serves as a ligand that bind to a transmembrane protein called GPCR and initiates a sequence of cascading pathways further downstream, that eventually results in the release of glucose into the blood to support the response we carry out. | Within the course of our daily activities, we experience several situations that cause us to jump on our feet and also, initiate some kind of response to these situations. Our bodies have an outstanding way of preparing us for such events as to whether flee or fight and it does this by making energy in the form of glucose available in the blood to support our reactions to these events. Our response is effectively mediated through reflex actions which are propagated through networks of neurons that are wired all over our body and eventually to the brain. One of the neurotransmitters produced through the coordination of these neuronal networks is epinephrine. Epinephrine serves as a ligand that bind to a transmembrane protein called GPCR and initiates a sequence of cascading pathways further downstream, that eventually results in the release of glucose into the blood to support the response we carry out. | ||
These ligands bind to a receptor in the Transmembrane called GPCRs. Once the ligand is able to bind tightly to the receptor, the first committed step of the pathway is initiated and the connected pieces begin to join in together until blood glucose level is replenished. More than 800 genes code for GPCRs that regulate several signaling pathways which control blood pressure, behavior, sleep, immune response, cognition…and many other cellular processes. | These ligands bind to a receptor in the Transmembrane called GPCRs. Once the ligand is able to bind tightly to the receptor, the first committed step of the pathway is initiated and the connected pieces begin to join in together until blood glucose level is replenished. More than 800 genes code for GPCRs that regulate several signaling pathways which control blood pressure, behavior, sleep, immune response, cognition…and many other cellular processes. | ||
| + | |||
| Line 49: | Line 48: | ||
[[Image:Venus.png|thumb|upright=1.5| Venus fly-trap position of the binding pocket]] | [[Image:Venus.png|thumb|upright=1.5| Venus fly-trap position of the binding pocket]] | ||
| - | [[Image:Terminal.png|thumb|upleft=2.0| | + | [[Image:Terminal.png|thumb|upleft=2.0| Amino and Carboxyl terminal groups]] |
| Line 61: | Line 60: | ||
| - | + | == FUNCTIONS == | |
| - | GPCRs act as a bridge and communicate the conditions of the cell to the nucleus to induce transcription or affect the processivity of catalytic pathway by activating certain enzymes. In the event of fight/flight, upon binding the ligand epinephrine, it causes a conformational change that leads to the exchange of the Guanine nucleotide (GDP to GTP) and that mechanism "turns" the switch on. The | + | GPCRs act as a bridge and communicate the conditions of the cell to the nucleus to induce transcription or affect the processivity of catalytic pathway by activating certain enzymes. In the event of fight/flight, upon binding the ligand epinephrine, it causes a conformational change that leads to the exchange of the Guanine nucleotide (GDP to GTP) and that mechanism "turns" the switch on. The alpha subunit then dissociate from the beta and gamma subunits and activates adenylate cyclase which makes cAMP. The cAMP activates Protein Kinase A by binding to its regulatory subunits, and that releases the catalytic subunits to further phosphorylate other proteins in the cell. Eventually, Glycogen phosphorylase is activated which breaks down glucose for use within the cell. |
GPCRs control many process which includes the dopaminergic pathway (involved in reward and learning), controls heart rate, vision, metabolism etc. | GPCRs control many process which includes the dopaminergic pathway (involved in reward and learning), controls heart rate, vision, metabolism etc. | ||
| Line 79: | Line 78: | ||
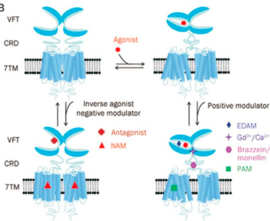
The kind and specificity of the agonists determine the activation pathway taken by GPCRs in terms of the energy ordering of the different states which is also premeditated by the respective activation energy landscapes. This means that the agonist-bound inactive state will have the lowest energy state among the various states within the degree of activation. | The kind and specificity of the agonists determine the activation pathway taken by GPCRs in terms of the energy ordering of the different states which is also premeditated by the respective activation energy landscapes. This means that the agonist-bound inactive state will have the lowest energy state among the various states within the degree of activation. | ||
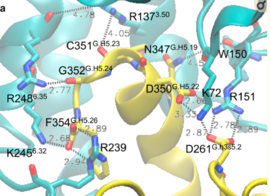
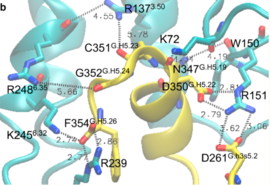
| - | Activation of the G-protein can be explained by interactions between the GPCR (specifically the ICD) and the G-alpha subunit. During analysis, Molecular Dynamics (MD) simulations found that there is formation of Salt-bridges and Hydrogen bonds between the C-terminal helix of G∝i and some regions in the ICD. Salt-bridges form between D261, D350 of G∝i and K72 of the IC loop 1 and R51 of IC loop 2.<ref>PMID: 27332120</ref> There's also formation of Hydrogen bonds between C351 and R137 of the G∝i that replace the inactive state R151-D136-R137-T247 network.Another salt-bridge is formed between the carboxylate group on the G∝i C-terminal residue F354 and K245 of the TM6 and R239 of the IC loop 3. All these structural changes facilitate ligand binding to receptor with high affinity and specificity and leads to an ordered state that further accommodates a more tighter binding to the ligand. The ICD then release the D/E R Y sequence motif from the TMD and this process is coupled to the binding of a ligand that is specific to the coupled domain (G-protein).Full activation occurs when G-proteins bind to the ICD. The stabilizing factor of the activated state of the GPCRs is attributed to the interactions between the Ga (G alpha subunit) and the GPCR. | + | Activation of the G-protein can be explained by interactions between the GPCR (specifically the ICD) and the G-alpha subunit. During analysis, Molecular Dynamics (MD) simulations found that there is formation of Salt-bridges and Hydrogen bonds between the C-terminal helix of G∝i and some regions in the ICD. Salt-bridges form between D261, D350 of G∝i and K72 of the IC loop 1 and R51 of IC loop 2.<ref name="verba">PMID:27332120</ref> There's also formation of Hydrogen bonds between C351 and R137 of the G∝i that replace the inactive state R151-D136-R137-T247 network.Another salt-bridge is formed between the carboxylate group on the G∝i C-terminal residue F354 and K245 of the TM6 and R239 of the IC loop 3. All these structural changes facilitate ligand binding to receptor with high affinity and specificity and leads to an ordered state that further accommodates a more tighter binding to the ligand. The ICD then release the D/E R Y sequence motif from the TMD and this process is coupled to the binding of a ligand that is specific to the coupled domain (G-protein).Full activation occurs when G-proteins bind to the ICD. The stabilizing factor of the activated state of the GPCRs is attributed to the interactions between the Ga (G alpha subunit) and the GPCR. |
'''DISEASE''' | '''DISEASE''' | ||
| - | Many GPCRs have become drug targets of many pharmaceutical drugs because of their central role in controlling many metabolic activities in the cell. Diseases including cancer, diabetes, | + | Many GPCRs have become drug targets of many pharmaceutical drugs because of their central role in controlling many metabolic activities in the cell. Diseases including cancer, diabetes, Alzheimer's, sleep deprivation and many others are treated by exploiting the druggability of GPCRs and how they mediate their responses to hormones, cytokines, and neurotransmitters. Of all drugs on the market that have been |
approved by the CDC, about 35% elicit their potency and mediate their effects through GPCRs. | approved by the CDC, about 35% elicit their potency and mediate their effects through GPCRs. | ||
| + | |||
| + | |||
| + | |||
| + | == References == | ||
| + | <references/> | ||
Revision as of 17:14, 10 December 2018
| This Sandbox is Reserved from November 5 2018 through January 1, 2019 for use in the course "CHEM 4923: Senior Project taught by Christina R. Bourne at the University of Oklahoma, Norman, USA. This reservation includes Sandbox Reserved 1471 through Sandbox Reserved 1478. |
To get started:
More help: Help:Editing |
This page is reserved for Manny
Your Heading Here (maybe something like 'Structure')
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
THE GENUIS OF GPCRs
|
| |||||||||||