Sandbox Reserved 1473
From Proteopedia
| Line 10: | Line 10: | ||
Within the course of our daily activities, we experience several situations that cause us to jump on our feet and also, initiate some kind of response to these situations. Our bodies have an outstanding way of preparing us for such events as to whether flee or fight and it does this by making energy in the form of glucose available in the blood to support our reactions to these events. Our response is effectively mediated through reflex actions which are propagated through networks of neurons that are wired all over our body and eventually to the brain. One of the neurotransmitters produced through the coordination of these neuronal networks is epinephrine. Epinephrine serves as a ligand that bind to a transmembrane protein called GPCR and initiates a sequence of cascading pathways further downstream, that eventually results in the release of glucose into the blood to support the response we carry out. | Within the course of our daily activities, we experience several situations that cause us to jump on our feet and also, initiate some kind of response to these situations. Our bodies have an outstanding way of preparing us for such events as to whether flee or fight and it does this by making energy in the form of glucose available in the blood to support our reactions to these events. Our response is effectively mediated through reflex actions which are propagated through networks of neurons that are wired all over our body and eventually to the brain. One of the neurotransmitters produced through the coordination of these neuronal networks is epinephrine. Epinephrine serves as a ligand that bind to a transmembrane protein called GPCR and initiates a sequence of cascading pathways further downstream, that eventually results in the release of glucose into the blood to support the response we carry out. | ||
| - | These ligands bind to a receptor in the Transmembrane called GPCRs. Once the ligand is able to bind tightly to the receptor, the first committed step of the pathway is initiated and the connected pieces begin to join in together until blood glucose level is replenished. There are about 360 pharmaceutically relevant GPCRs with the human genome and, more than 800 genes code for GPCRs that regulate several signaling pathways which control blood pressure, behavior, sleep, immune response, cognition…and many other cellular processes<ref name="Brogi">PMID: 25506327</ref>. | + | These ligands bind to a receptor in the Transmembrane called GPCRs. Once the ligand is able to bind tightly to the receptor, the first committed step of the pathway is initiated and the connected pieces begin to join in together until blood glucose level is replenished<ref name="Dong">PMID: 27332120</ref>. There are about 360 pharmaceutically relevant GPCRs with the human genome and, more than 800 genes code for GPCRs that regulate several signaling pathways which control blood pressure, behavior, sleep, immune response, cognition…and many other cellular processes<ref name="Brogi">PMID: 25506327</ref>. |
| Line 19: | Line 19: | ||
[[Image:Disulfide Linkages.png|thumb|downright=2.0| Figure 1:Disulfide Linkages between the TMD and ECL (taken from Google)]] | [[Image:Disulfide Linkages.png|thumb|downright=2.0| Figure 1:Disulfide Linkages between the TMD and ECL (taken from Google)]] | ||
[[Image:All.png|thumb|upright=1.5| Figure 2:Showing all the parts together (Source Unknown)]] | [[Image:All.png|thumb|upright=1.5| Figure 2:Showing all the parts together (Source Unknown)]] | ||
| - | [[Image:Terminal.png|thumb|upleft=2.0| Figure 3:Amino and Carboxyl terminal groups]] | + | [[Image:Terminal.png|thumb|upleft=2.0| Figure 3:Amino and Carboxyl terminal groups (taken from Google)]] |
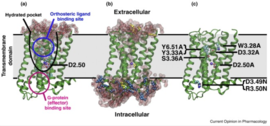
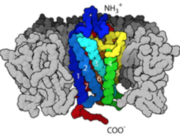
| - | G-Protein Coupled Receptors [[GPCR]], also known as 7 transmembrane receptors [because of its 7 constituent alpha helices] are the largest groups of transmembrane protein receptors in eukaryotes. There are many different classes GPCRs depending on the position of their binding sites or the kind of ligands they bind to. There are about six classes of GPCRs and they include the classes A, B, C, D, E, AND F. Beta adrenergic receptors fall the class C with other metabotropic hormones while Rhodopsin (the light sensing cells in our eye) are categorized under class A. All studied GPCRs have a conserved structure that can be subdivided into; the extracellular domain (ECD), which comprises the three extracellular loops (EC1), (EC2), (EC3) and the N-terminal amino groups, the seven spanning helices transmembrane domain (7TM) embedded within the plasma membrane, and the intracellular domains ICD which also comprises the carboxyl-terminal residues and three intracellular loops (ICL1, ICL2, ICL3). The binding of the receptor to a ligand causes a structural change in the intracellular domains that also signals/activates the alpha subunit of a G-protein to exchange GDP for GTP, transducing the signal further downstream. | + | G-Protein Coupled Receptors [[GPCR]], also known as 7 transmembrane receptors [because of its 7 constituent alpha helices] are the largest groups of transmembrane protein receptors in eukaryotes. There are many different classes GPCRs depending on the position of their binding sites or the kind of ligands they bind to. There are about six classes of GPCRs and they include the classes A, B, C, D, E, AND F<ref name="Harmar" />. Beta adrenergic receptors fall the class C with other metabotropic hormones while Rhodopsin (the light sensing cells in our eye) are categorized under class A. All studied GPCRs have a conserved structure that can be subdivided into; the extracellular domain (ECD), which comprises the three extracellular loops (EC1), (EC2), (EC3) and the N-terminal amino groups(Figure:3), the seven spanning helices transmembrane domain (7TM) embedded within the plasma membrane, and the intracellular domains ICD which also comprises the carboxyl-terminal residues and three intracellular loops (ICL1, ICL2, ICL3)<ref name="Harmar">PMID: 11790261</ref> <ref name="Unal">PMID: 22037017</ref>. The binding of the receptor to a ligand causes a structural change in the intracellular domains that also signals/activates the alpha subunit of a G-protein to exchange GDP for GTP, transducing the signal further downstream(Figure 2). |
| - | For some GPCRs, the ligand binds to a site in the 7TM (certain class A) or both the ECD and the 7TM as in certain class B GPCRs. However, in the Beta Adrenergic receptor, the ligand binds at the the <scene name='80/800652/Showing_ligand/1'>extracellular domain [ECD]</scene> which acts like a fly-trap pocket. The transmembrane domain is structurally coupled to the extracellular domain through Disulfide linkages and mutations within this region disrupts the entire structure and function of GPCRs. | + | For some GPCRs, the ligand binds to a site in the 7TM (certain class A) or both the ECD and the 7TM as in certain class B GPCRs. However, in the Beta Adrenergic receptor, the ligand binds at the the <scene name='80/800652/Showing_ligand/1'>extracellular domain [ECD]</scene> which acts like a fly-trap pocket. The transmembrane domain is structurally coupled to the extracellular domain through Disulfide linkages (Figure: 1) and mutations within this region disrupts the entire structure and function of GPCRs<ref name="Brogi" /> <ref name="Salon" /> . |
| - | The intra cellular domain sits in the cytoplasmic end of the transmembrane domain. This region contains the D/E R Y motif; a conserved region within the ICD that interacts with interacts with intracellular ligands like the G-subunit (activates | + | The intra cellular domain sits in the cytoplasmic end of the transmembrane domain. This region contains the D/E R Y motif; a conserved region within the ICD that interacts with interacts with intracellular ligands like the G-subunit and Beta Arrestins (activates them), and phosphorylate other kinases as well<ref name="Salon">PMID: 21969326</ref> <ref name="Peterson">PMID: 28626043</ref>. The C-terminal domain is structurally independent of the ECD and can adopt functionally discrete conformation in the absence of transmembrane helices. The C-terminal domain regulates the interactions with cytosolic proteins involved in signaling. |
| Line 36: | Line 36: | ||
Many GPCRs have become drug targets of many pharmaceutical drugs because of their central role in controlling many metabolic activities in the cell. Diseases including cancer, diabetes, Alzheimer's, sleep deprivation and many others are treated by exploiting the druggability of GPCRs and how they mediate their responses to hormones, cytokines, and neurotransmitters. Of all drugs on the market that have been | Many GPCRs have become drug targets of many pharmaceutical drugs because of their central role in controlling many metabolic activities in the cell. Diseases including cancer, diabetes, Alzheimer's, sleep deprivation and many others are treated by exploiting the druggability of GPCRs and how they mediate their responses to hormones, cytokines, and neurotransmitters. Of all drugs on the market that have been | ||
| - | approved by the CDC, about 35% elicit their potency and mediate their effects through GPCRs<ref name="Basith">PMID: 29593527</ref>. The most recently FDA approved GPCR-targeted drug is Zilretta which is a glucocorticoid receptor agonist that is used for the pain management of knee osteoarthritis.<ref name="Basith" / | + | approved by the CDC, about 35% elicit their potency and mediate their effects through GPCRs<ref name="Basith">PMID: 29593527</ref>. The most recently FDA approved GPCR-targeted drug is Zilretta which is a glucocorticoid receptor agonist that is used for the pain management of knee osteoarthritis.<ref name="Basith" /> |
| - | + | ||
| - | + | ||
| + | GPCRs control many process which includes; the dopaminergic pathway that is involved in a series of neurological and psychiatric diseases such as Parkinson’s disease, bipolar disorder, drug addiction, schizophrenia, and Huntington’s disease. GPCRs also control important functions such as controls heart rate, vision, metabolism, obesity, diabetes, etc<ref name="Brogi" />. | ||
| Line 45: | Line 44: | ||
---- | ---- | ||
| - | [[Image:Energetics1.png|thumb|upright=1.5|Figure | + | [[Image:Energetics1.png|thumb|upright=1.5|Figure 4:Salt bridge formation (PUBMED id PMID: 27332120)]] |
| - | [[Image:Energetics2.png|thumb|downright=1.5|Figure | + | [[Image:Energetics2.png|thumb|downright=1.5|Figure 5:Hydrogen bond formation (PUBMED id PMID: 27332120)]] |
| - | Experimental studies have shown that GPCRs do trigger cellular activities in their unbound states at a minimum basal levels. Studies have also shown that even when bound to agonists, GPCRs express characteristics that range from inactive to a continuum of partially active structures in the absence of the G protein. This means that the presence of the agonist plays an important role in the activation of GPCRs. | + | Experimental studies have shown that GPCRs do trigger cellular activities in their unbound states at a minimum basal levels. Studies have also shown that even when bound to agonists, GPCRs express characteristics that range from inactive to a continuum of partially active structures in the absence of the G protein<ref name="Dong" />. This means that the presence of the agonist plays an important role in the activation of GPCRs.The kind and specificity of the agonists determine the activation pathway taken by GPCRs in terms of the energy ordering of the different states which is also premeditated by the respective activation energy landscapes. This means that the agonist-bound inactive state will have the lowest energy state among the various states within the degree of activation<ref name="Dong" />. |
| - | The kind and specificity of the agonists determine the activation pathway taken by GPCRs in terms of the energy ordering of the different states which is also premeditated by the respective activation energy landscapes. This means that the agonist-bound inactive state will have the lowest energy state among the various states within the degree of activation. | + | |
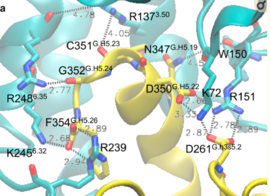
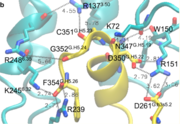
| - | Activation of the G-protein can be explained by interactions between the GPCR (specifically the ICD) and the G-alpha subunit. During analysis, Molecular Dynamics (MD) simulations found that there is formation of Salt-bridges and Hydrogen bonds between the C-terminal helix of G∝i and some regions in the ICD. Salt-bridges form between D261, D350 of G∝i and K72 of the IC loop 1 and R51 of IC loop 2 | + | Activation of the G-protein can be explained by interactions between the GPCR (specifically the ICD) and the G-alpha subunit. During analysis, Molecular Dynamics (MD) simulations found that there is formation of Salt-bridges and Hydrogen bonds between the C-terminal helix of G∝i and some regions in the ICD. Salt-bridges form between D261, D350 of G∝i and K72 of the IC loop 1 and R51 of IC loop 2(Figure 4)<ref name="Dong" />. There's also formation of Hydrogen bonds between C351 and R137 of the G∝i that replace the inactive state R151-D136-R137-T247 network<ref name="Dong" />.Another salt-bridge is formed between the carboxylate group on the G∝i C-terminal residue F354 and K245 of the TM6 and R239 of the IC loop 3(Figure 5)<ref name="Dong" />. All these structural changes facilitate ligand binding to receptor with high affinity and specificity and leads to an ordered state that further accommodates a more tighter binding to the ligand. The ICD then release the D/E R Y sequence motif from the TMD and this process is coupled to the binding of a ligand that is specific to the coupled domain (G-protein).Full activation occurs when G-proteins bind to the ICD. The stabilizing factor of the activated state of the GPCRs is attributed to the interactions between the Ga (G alpha subunit) and the GPCR<ref name="Dong" />. |
Current revision
| This Sandbox is Reserved from November 5 2018 through January 1, 2019 for use in the course "CHEM 4923: Senior Project taught by Christina R. Bourne at the University of Oklahoma, Norman, USA. This reservation includes Sandbox Reserved 1471 through Sandbox Reserved 1478. |
To get started:
More help: Help:Editing |
This page is reserved for Manny
Contents |
THE GENUIS OF GPCRs
INTRODUCTION
|
Within the course of our daily activities, we experience several situations that cause us to jump on our feet and also, initiate some kind of response to these situations. Our bodies have an outstanding way of preparing us for such events as to whether flee or fight and it does this by making energy in the form of glucose available in the blood to support our reactions to these events. Our response is effectively mediated through reflex actions which are propagated through networks of neurons that are wired all over our body and eventually to the brain. One of the neurotransmitters produced through the coordination of these neuronal networks is epinephrine. Epinephrine serves as a ligand that bind to a transmembrane protein called GPCR and initiates a sequence of cascading pathways further downstream, that eventually results in the release of glucose into the blood to support the response we carry out.
These ligands bind to a receptor in the Transmembrane called GPCRs. Once the ligand is able to bind tightly to the receptor, the first committed step of the pathway is initiated and the connected pieces begin to join in together until blood glucose level is replenished[1]. There are about 360 pharmaceutically relevant GPCRs with the human genome and, more than 800 genes code for GPCRs that regulate several signaling pathways which control blood pressure, behavior, sleep, immune response, cognition…and many other cellular processes[2].
STRUCTURE HIGHLIGHTS
Structural components of the B2 GPCR.
G-Protein Coupled Receptors GPCR, also known as 7 transmembrane receptors [because of its 7 constituent alpha helices] are the largest groups of transmembrane protein receptors in eukaryotes. There are many different classes GPCRs depending on the position of their binding sites or the kind of ligands they bind to. There are about six classes of GPCRs and they include the classes A, B, C, D, E, AND F[3]. Beta adrenergic receptors fall the class C with other metabotropic hormones while Rhodopsin (the light sensing cells in our eye) are categorized under class A. All studied GPCRs have a conserved structure that can be subdivided into; the extracellular domain (ECD), which comprises the three extracellular loops (EC1), (EC2), (EC3) and the N-terminal amino groups(Figure:3), the seven spanning helices transmembrane domain (7TM) embedded within the plasma membrane, and the intracellular domains ICD which also comprises the carboxyl-terminal residues and three intracellular loops (ICL1, ICL2, ICL3)[3] [4]. The binding of the receptor to a ligand causes a structural change in the intracellular domains that also signals/activates the alpha subunit of a G-protein to exchange GDP for GTP, transducing the signal further downstream(Figure 2).
For some GPCRs, the ligand binds to a site in the 7TM (certain class A) or both the ECD and the 7TM as in certain class B GPCRs. However, in the Beta Adrenergic receptor, the ligand binds at the the which acts like a fly-trap pocket. The transmembrane domain is structurally coupled to the extracellular domain through Disulfide linkages (Figure: 1) and mutations within this region disrupts the entire structure and function of GPCRs[2] [5] .
The intra cellular domain sits in the cytoplasmic end of the transmembrane domain. This region contains the D/E R Y motif; a conserved region within the ICD that interacts with interacts with intracellular ligands like the G-subunit and Beta Arrestins (activates them), and phosphorylate other kinases as well[5] [6]. The C-terminal domain is structurally independent of the ECD and can adopt functionally discrete conformation in the absence of transmembrane helices. The C-terminal domain regulates the interactions with cytosolic proteins involved in signaling.
FUNCTION
GPCRs act as a bridge and communicate the conditions of the cell to the nucleus to induce transcription or affect the processivity of catalytic pathway by activating certain enzymes. In the event of fight/flight, upon binding the ligand epinephrine, it causes a conformational change that leads to the exchange of the Guanine nucleotide (GDP to GTP) and that mechanism "turns" the switch on. The alpha subunit then dissociate from the beta and gamma subunits and activates adenylate cyclase which makes cAMP. The cAMP activates Protein Kinase A by binding to its regulatory subunits, and that releases the catalytic subunits to further phosphorylate other proteins in the cell. Eventually, Glycogen phosphorylase is activated which breaks down glucose for use within the cell.
DISEASE AND DRUGGABILITY
Many GPCRs have become drug targets of many pharmaceutical drugs because of their central role in controlling many metabolic activities in the cell. Diseases including cancer, diabetes, Alzheimer's, sleep deprivation and many others are treated by exploiting the druggability of GPCRs and how they mediate their responses to hormones, cytokines, and neurotransmitters. Of all drugs on the market that have been approved by the CDC, about 35% elicit their potency and mediate their effects through GPCRs[7]. The most recently FDA approved GPCR-targeted drug is Zilretta which is a glucocorticoid receptor agonist that is used for the pain management of knee osteoarthritis.[7]
GPCRs control many process which includes; the dopaminergic pathway that is involved in a series of neurological and psychiatric diseases such as Parkinson’s disease, bipolar disorder, drug addiction, schizophrenia, and Huntington’s disease. GPCRs also control important functions such as controls heart rate, vision, metabolism, obesity, diabetes, etc[2].
ENERGETICS
Experimental studies have shown that GPCRs do trigger cellular activities in their unbound states at a minimum basal levels. Studies have also shown that even when bound to agonists, GPCRs express characteristics that range from inactive to a continuum of partially active structures in the absence of the G protein[1]. This means that the presence of the agonist plays an important role in the activation of GPCRs.The kind and specificity of the agonists determine the activation pathway taken by GPCRs in terms of the energy ordering of the different states which is also premeditated by the respective activation energy landscapes. This means that the agonist-bound inactive state will have the lowest energy state among the various states within the degree of activation[1].
Activation of the G-protein can be explained by interactions between the GPCR (specifically the ICD) and the G-alpha subunit. During analysis, Molecular Dynamics (MD) simulations found that there is formation of Salt-bridges and Hydrogen bonds between the C-terminal helix of G∝i and some regions in the ICD. Salt-bridges form between D261, D350 of G∝i and K72 of the IC loop 1 and R51 of IC loop 2(Figure 4)[1]. There's also formation of Hydrogen bonds between C351 and R137 of the G∝i that replace the inactive state R151-D136-R137-T247 network[1].Another salt-bridge is formed between the carboxylate group on the G∝i C-terminal residue F354 and K245 of the TM6 and R239 of the IC loop 3(Figure 5)[1]. All these structural changes facilitate ligand binding to receptor with high affinity and specificity and leads to an ordered state that further accommodates a more tighter binding to the ligand. The ICD then release the D/E R Y sequence motif from the TMD and this process is coupled to the binding of a ligand that is specific to the coupled domain (G-protein).Full activation occurs when G-proteins bind to the ICD. The stabilizing factor of the activated state of the GPCRs is attributed to the interactions between the Ga (G alpha subunit) and the GPCR[1].
REFERENCES
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Dong SS, Goddard WA 3rd, Abrol R. Conformational and Thermodynamic Landscape of GPCR Activation from Theory and Computation. Biophys J. 2016 Jun 21;110(12):2618-2629. doi: 10.1016/j.bpj.2016.04.028. PMID:27332120 doi:http://dx.doi.org/10.1016/j.bpj.2016.04.028
- ↑ 2.0 2.1 2.2 Brogi S, Tafi A, Desaubry L, Nebigil CG. Discovery of GPCR ligands for probing signal transduction pathways. Front Pharmacol. 2014 Nov 28;5:255. doi: 10.3389/fphar.2014.00255. eCollection, 2014. PMID:25506327 doi:http://dx.doi.org/10.3389/fphar.2014.00255
- ↑ 3.0 3.1 Harmar AJ. Family-B G-protein-coupled receptors. Genome Biol. 2001;2(12):REVIEWS3013. Epub 2001 Nov 23. PMID:11790261
- ↑ Unal H, Karnik SS. Domain coupling in GPCRs: the engine for induced conformational changes. Trends Pharmacol Sci. 2012 Feb;33(2):79-88. doi: 10.1016/j.tips.2011.09.007. Epub, 2011 Oct 29. PMID:22037017 doi:http://dx.doi.org/10.1016/j.tips.2011.09.007
- ↑ 5.0 5.1 Salon JA, Lodowski DT, Palczewski K. The significance of G protein-coupled receptor crystallography for drug discovery. Pharmacol Rev. 2011 Dec;63(4):901-37. doi: 10.1124/pr.110.003350. PMID:21969326 doi:http://dx.doi.org/10.1124/pr.110.003350
- ↑ Peterson YK, Luttrell LM. The Diverse Roles of Arrestin Scaffolds in G Protein-Coupled Receptor Signaling. Pharmacol Rev. 2017 Jul;69(3):256-297. doi: 10.1124/pr.116.013367. PMID:28626043 doi:http://dx.doi.org/10.1124/pr.116.013367
- ↑ 7.0 7.1 Basith S, Cui M, Macalino SJY, Park J, Clavio NAB, Kang S, Choi S. Exploring G Protein-Coupled Receptors (GPCRs) Ligand Space via Cheminformatics Approaches: Impact on Rational Drug Design. Front Pharmacol. 2018 Mar 9;9:128. doi: 10.3389/fphar.2018.00128. eCollection, 2018. PMID:29593527 doi:http://dx.doi.org/10.3389/fphar.2018.00128