User:Gisele A. Andree/Sandbox 1
From Proteopedia
(Difference between revisions)
(New page: ==Structure of Eukaryotic Dihydropyrimidine Dehydrogenase == <StructureSection load='1stp' size='340' side='right' caption='Caption for this structure' scene=''> This is a default text for...) |
|||
| Line 7: | Line 7: | ||
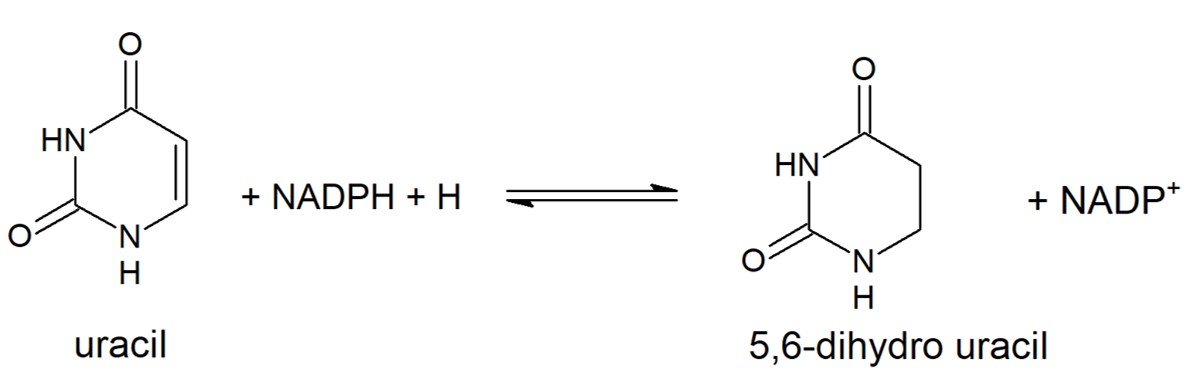
Dihydropyrimidine Dehydrogenase (DPD) is the first enzyme in pyrimidine degradation pathway. It catalyzes the reduction of 5,6-double bond to obtain dihydropyrimidine. | Dihydropyrimidine Dehydrogenase (DPD) is the first enzyme in pyrimidine degradation pathway. It catalyzes the reduction of 5,6-double bond to obtain dihydropyrimidine. | ||
| - | [[Image: | + | [[Image:Uracil_reduction.jpg]] |
| + | Reduction of Uracil to form 5,6-dihydro uracil. This reaction is catalyzed by eukaryotic DPD. Other pyrimidines can take the place of uracil in this reaction and will be metabolized in the same way. | ||
== Disease == | == Disease == | ||
| + | 5-fluorouracil (5-FU) is a drug used to treat a variety of cancers as it has wide anti-tumor activity & works well alongside other chemotherapy drugs. In the human liver 80-85% of 5-FU is catabolized into inactive, and potentially toxic, metabolites by DPD. Only 1-3% of the original dose proceeds through anabolic pathways to create active cytotoxic complexes. The active complexes inhibit DNA synthesis and the processing and function of RNA processing thus producing a deleterious effect on both healthy and cancerous cells. | ||
| + | |||
| + | DPD decreases effectivity of drug thus requires a very high dosages, leading to major side effects. Luckily, inhibitors are in development and some are in clinical trials. | ||
| + | |||
== Relevance == | == Relevance == | ||
Revision as of 19:40, 13 December 2018
Structure of Eukaryotic Dihydropyrimidine Dehydrogenase
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644