14-3-3 protein
From Proteopedia
(Difference between revisions)
| Line 15: | Line 15: | ||
== Structural highlights == | == Structural highlights == | ||
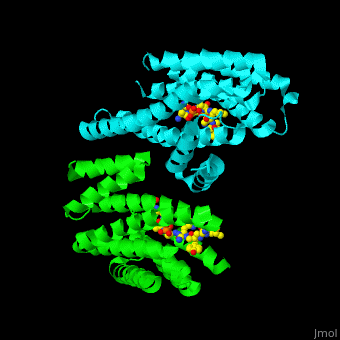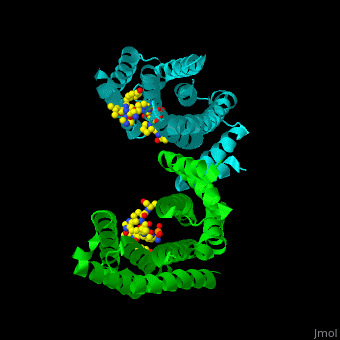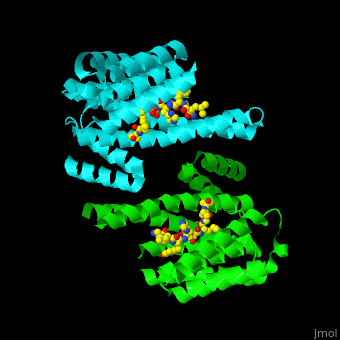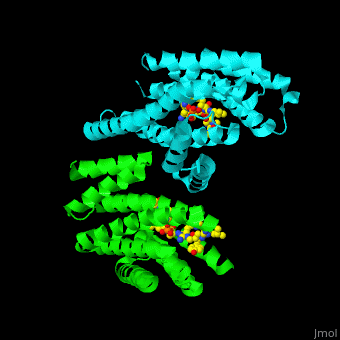
| - | PRS are homo- and heterodimers containing <scene name='59/590827/Cv/ | + | PRS are homo- and heterodimers containing <scene name='59/590827/Cv/14'>9 antiparallel α-helices</scene>. <scene name='59/590827/Cv/15'>Three of the helices form the dimerization domain</scene> (<font color='red'><b>3 helices of chain A are in red</b></font> and <font color='magenta'><b>3 helices of chain B are in magenta</b></font>). <scene name='59/590827/Cv/16'>Five residues (in PRS-σ and PRS-ζ) are involved in ligand binding</scene>. |
</StructureSection> | </StructureSection> | ||
Revision as of 11:19, 16 December 2018
| |||||||||||
3D structures of 14-3-3 proteins (Updated on 16-December-2018)
References
- ↑ Benzinger A, Popowicz GM, Joy JK, Majumdar S, Holak TA, Hermeking H. The crystal structure of the non-liganded 14-3-3sigma protein: insights into determinants of isoform specific ligand binding and dimerization. Cell Res. 2005 Apr;15(4):219-27. PMID:15857576 doi:10.1038/sj.cr.7290290