We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1481
From Proteopedia
(Difference between revisions)
| Line 25: | Line 25: | ||
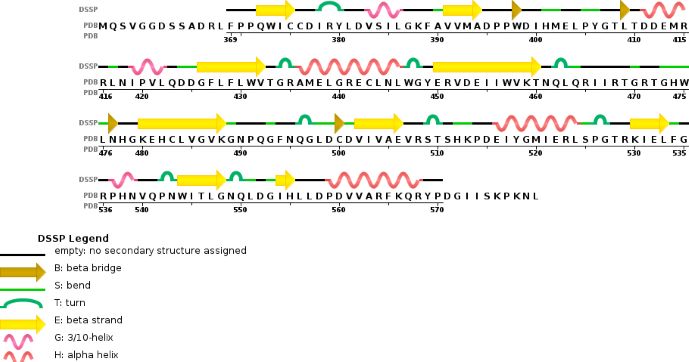
Chain A METTL3'secondary structure, is mainly composed of 20% helical (7 helices; 46 residues)and 24% beta sheet (13 strands; 55 residues) | Chain A METTL3'secondary structure, is mainly composed of 20% helical (7 helices; 46 residues)and 24% beta sheet (13 strands; 55 residues) | ||
| - | [[Image:chain 1 METTL3.jpg]] | + | [[Image:chain 1 METTL3.jpg]] |
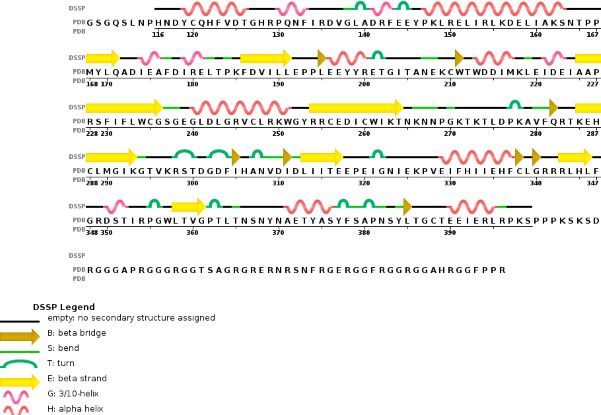
Chain B METTL14 is made of 24% helical s (14 helices; 87 residues) and 18% beta sheet (16 strands; 63 residues) | Chain B METTL14 is made of 24% helical s (14 helices; 87 residues) and 18% beta sheet (16 strands; 63 residues) | ||
| - | [[Image:chain 2 METTL14.jpg]] | + | [[Image:chain 2 METTL14.jpg]] |
'''Tertiary structure''' | '''Tertiary structure''' | ||
Revision as of 14:29, 29 December 2018
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
Crystal structure of the catalytic domains of Mettl3/Mettl14 complexInsert caption here
Drag the structure with the mouse to rotate
Insert caption here |
| Drag the structure with the mouse to rotate |
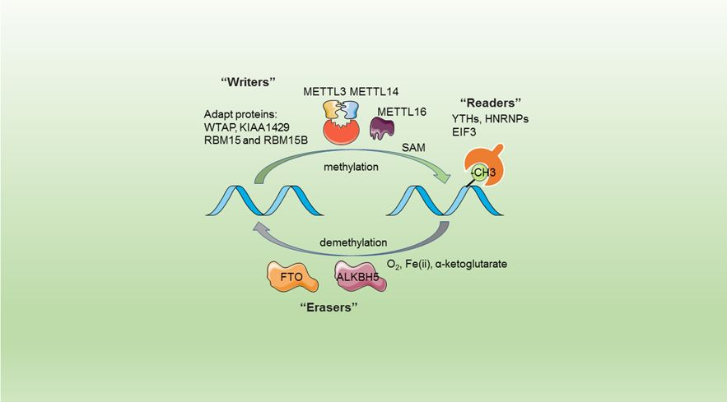
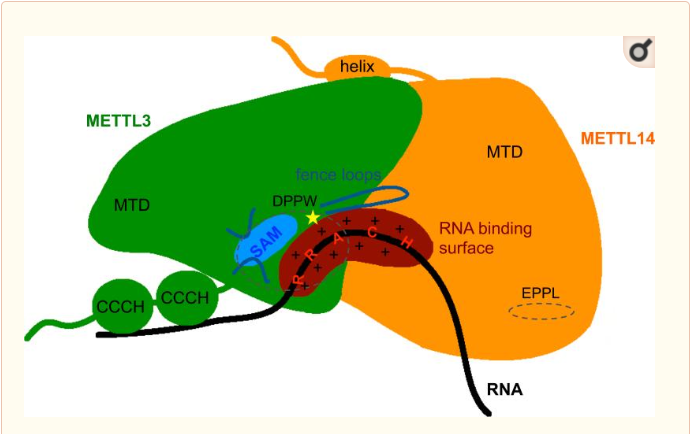
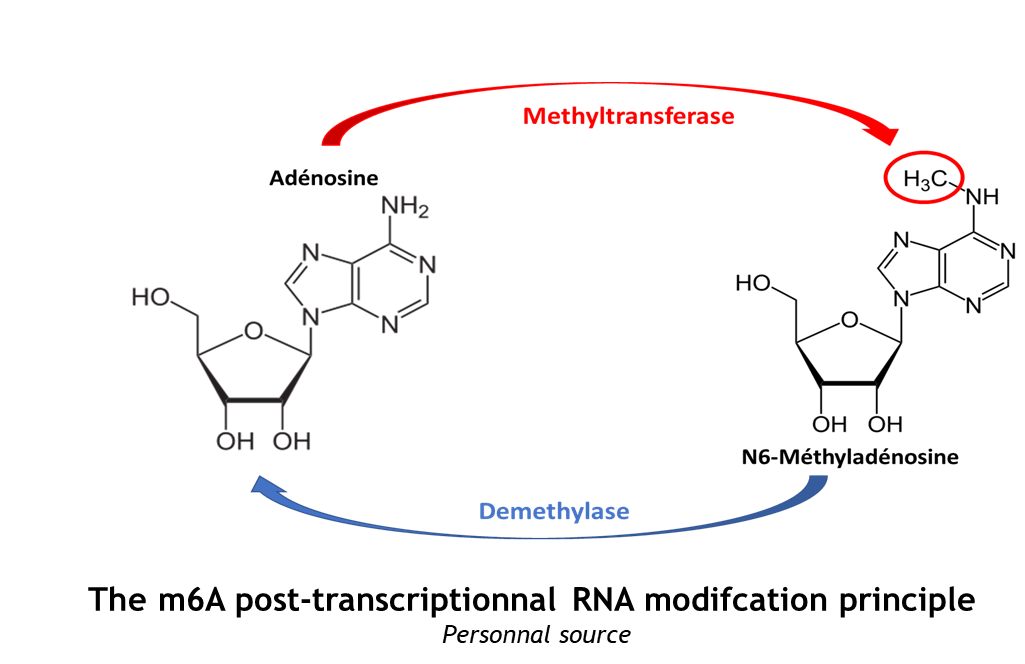
The complex METTL3/METTL14 is a heterodimer enzymatic complex involved into RNA post-transcription modifications by humans. This complex is abble to add a methyl group on adenosin of the RNA, by catalyzing a m6(A) modification.The N(6)-methyladenosine (m(6)A) is a quite common, reversible chemical modifications of RNAs molecules which plays a key role in several biological fonctions. This post transcriptional modification can be added by WRITERS, recognized by READERS and also removed byr ERASERS. The METTL3/METTL14 complex plays the role of writer.
| |||||||||||
References
- ↑ . PMID:216315890657
- ↑ . PMID:216315890657
- ↑ Wang P, Doxtader KA, Nam Y. Structural Basis for Cooperative Function of Mettl3 and Mettl14 Methyltransferases. Mol Cell. 2016 Jul 21;63(2):306-17. doi: 10.1016/j.molcel.2016.05.041. Epub 2016 , Jun 30. PMID:27373337 doi:http://dx.doi.org/10.1016/j.molcel.2016.05.041
- ↑ Wang X, Feng J, Xue Y, Guan Z, Zhang D, Liu Z, Gong Z, Wang Q, Huang J, Tang C, Zou T, Yin P. Structural basis of N(6)-adenosine methylation by the METTL3-METTL14 complex. Nature. 2016 May 25;534(7608):575-8. doi: 10.1038/nature18298. PMID:27281194 doi:http://dx.doi.org/10.1038/nature18298
- ↑ Sledz P, Jinek M. Structural insights into the molecular mechanism of the m(6)A writer complex. Elife. 2016 Sep 14;5. pii: e18434. doi: 10.7554/eLife.18434. PMID:27627798 doi:http://dx.doi.org/10.7554/eLife.18434
- ↑ Wang X, Huang J, Zou T, Yin P. Human m(6)A writers: Two subunits, 2 roles. RNA Biol. 2017 Mar 4;14(3):300-304. doi: 10.1080/15476286.2017.1282025. Epub 2017, Jan 25. PMID:28121234 doi:http://dx.doi.org/10.1080/15476286.2017.1282025