Sandbox Reserved 1481
From Proteopedia
| Line 32: | Line 32: | ||
'''Tertiary structure''' | '''Tertiary structure''' | ||
| + | Thanks to their primary amino acid sequences both A and B chains have some specific conserved domains directly linked to their function and functionning. | ||
'''Domains and specific site or sequences''' | '''Domains and specific site or sequences''' | ||
| - | Thanks to their primary amino acid sequences both A and B chains have some specific conserved domains directly linked to their function and functionning. | ||
'''MTD : Methyltransferase domain ''' | '''MTD : Methyltransferase domain ''' | ||
| Line 47: | Line 47: | ||
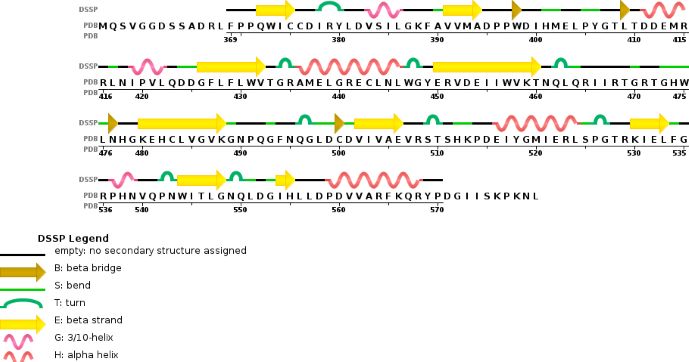
Zinc finger domain of the METTL3 N6-methyladenosine methyltransferase, is a RNA binding domain of the complex. This perticular domain of the two proteins complex allow the protein to make interactions with the RNA molecule to modify | Zinc finger domain of the METTL3 N6-methyladenosine methyltransferase, is a RNA binding domain of the complex. This perticular domain of the two proteins complex allow the protein to make interactions with the RNA molecule to modify | ||
[[Image:MET.PNG]] | [[Image:MET.PNG]] | ||
| + | |||
| + | |||
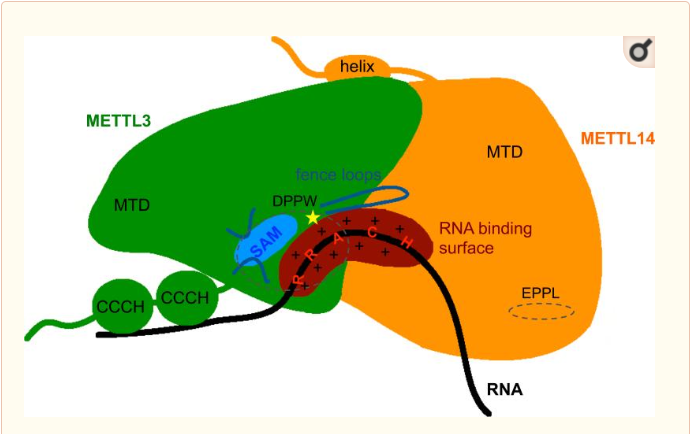
| + | METTL3 and METTL14 are linked to each other to form the functionnal complex. So as the METTL3 is responsible of the catalytic activity, the METTL14 allow the allosteric recognition of the RNA. The two subunit bindind creates a cavity in the center of the complex where the enzymatic reaction take place, it is the catalytic center. In these catalytic center some critical amino acid residues for the substrate recognition and specific binding are located. | ||
== Cristal structure == | == Cristal structure == | ||
Revision as of 15:41, 29 December 2018
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
Crystal structure of the catalytic domains of Mettl3/Mettl14 complexInsert caption here
Drag the structure with the mouse to rotate
Insert caption here |
| Drag the structure with the mouse to rotate |
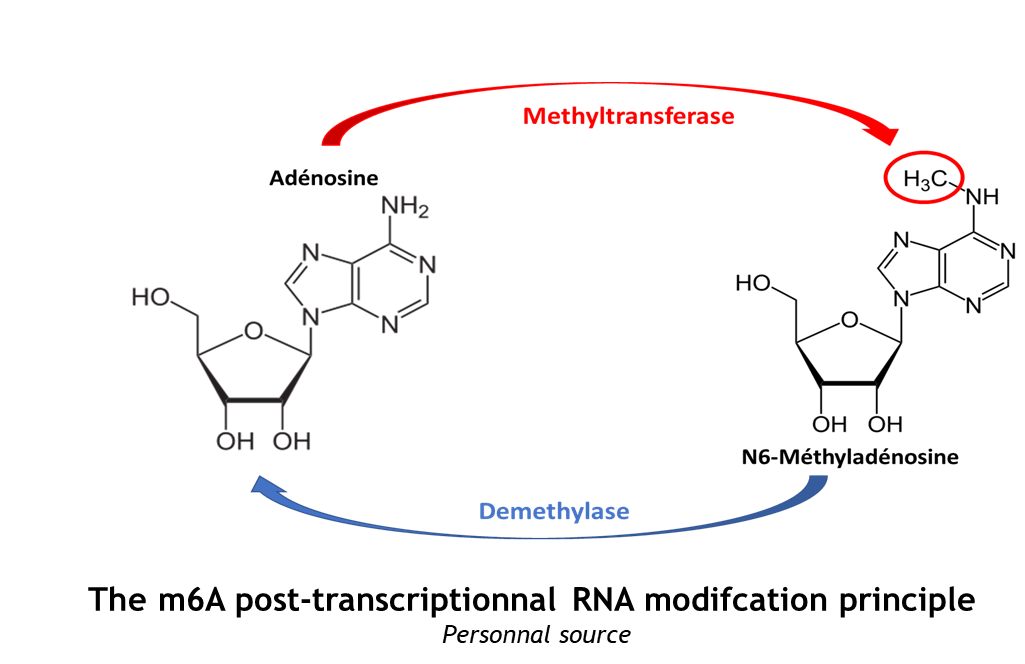
The complex METTL3/METTL14 is a heterodimer enzymatic complex involved into RNA post-transcription modifications by humans. This complex is abble to add a methyl group on adenosin of the RNA, by catalyzing a m6(A) modification.The N(6)-methyladenosine (m(6)A) is a quite common, reversible chemical modifications of RNAs molecules which plays a key role in several biological fonctions. This post transcriptional modification can be added by WRITERS, recognized by READERS and also removed byr ERASERS. The METTL3/METTL14 complex plays the role of writer.
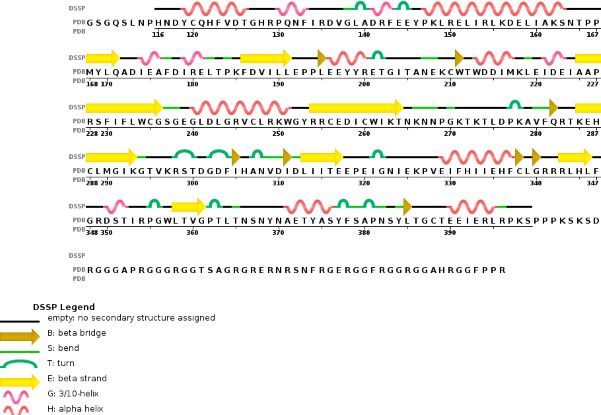
This enzymatic complex belongs to the second class of enzyme, which are the transferases. The complex is formed by 574 amino acid residues, divided into two different proteins nammed as Methyltransferase Like number 3 and 14. 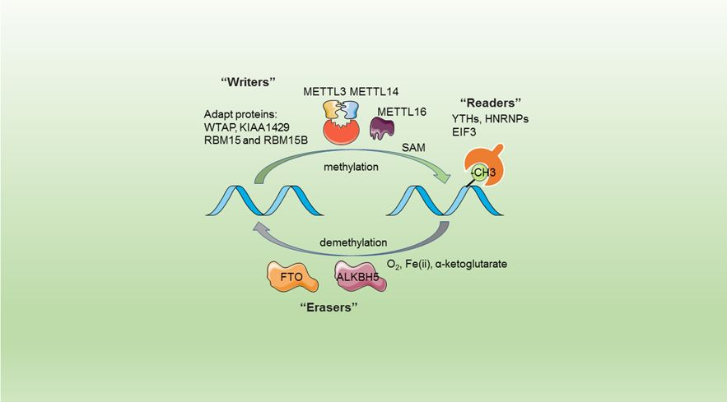
| |||||||||||
References
- ↑ . PMID:216315890657
- ↑ . PMID:216315890657
- ↑ Wang P, Doxtader KA, Nam Y. Structural Basis for Cooperative Function of Mettl3 and Mettl14 Methyltransferases. Mol Cell. 2016 Jul 21;63(2):306-17. doi: 10.1016/j.molcel.2016.05.041. Epub 2016 , Jun 30. PMID:27373337 doi:http://dx.doi.org/10.1016/j.molcel.2016.05.041
- ↑ Wang X, Feng J, Xue Y, Guan Z, Zhang D, Liu Z, Gong Z, Wang Q, Huang J, Tang C, Zou T, Yin P. Structural basis of N(6)-adenosine methylation by the METTL3-METTL14 complex. Nature. 2016 May 25;534(7608):575-8. doi: 10.1038/nature18298. PMID:27281194 doi:http://dx.doi.org/10.1038/nature18298
- ↑ Sledz P, Jinek M. Structural insights into the molecular mechanism of the m(6)A writer complex. Elife. 2016 Sep 14;5. pii: e18434. doi: 10.7554/eLife.18434. PMID:27627798 doi:http://dx.doi.org/10.7554/eLife.18434
- ↑ Wang X, Huang J, Zou T, Yin P. Human m(6)A writers: Two subunits, 2 roles. RNA Biol. 2017 Mar 4;14(3):300-304. doi: 10.1080/15476286.2017.1282025. Epub 2017, Jan 25. PMID:28121234 doi:http://dx.doi.org/10.1080/15476286.2017.1282025
- ↑ doi: https://dx.doi.org/10.2210/pdb5K7M/pdb