Sandbox Reserved 1481
From Proteopedia
| Line 1: | Line 1: | ||
{{Sandbox_Reserved_ESBS}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | {{Sandbox_Reserved_ESBS}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
==Crystal structure of the catalytic domains of Mettl3/Mettl14 complex<Structure load='5K7M' size='350' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' />== | ==Crystal structure of the catalytic domains of Mettl3/Mettl14 complex<Structure load='5K7M' size='350' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' />== | ||
| - | The complex METTL3/METTL14 is a heterodimer enzymatic complex involved into RNA post- | + | The complex METTL3/METTL14 is a heterodimer enzymatic complex involved into RNA post-transcriptional modifications by humans. |
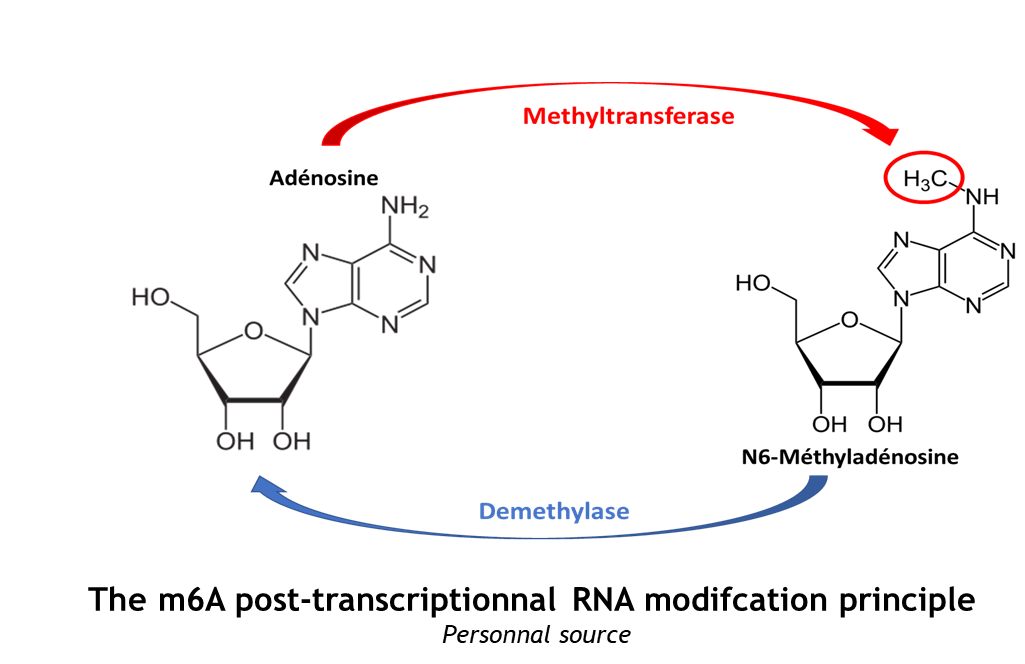
| - | This complex is abble to add a methyl group on adenosin of the RNA, by catalyzing a m6(A) modification.The N(6)-methyladenosine (m(6)A) is a quite common, reversible chemical | + | This complex is abble to add a methyl group on adenosin of the RNA, by catalyzing a m6(A) modification.The N(6)-methyladenosine (m(6)A) is a quite common, reversible chemical modification of RNAs molecules, which plays a key role in several different biological fonctions. This post-transcriptional modification can be added by WRITERS, recognized by READERS and also removed byr ERASERS. The METTL3/METTL14 complex plays the role of writer. |
This enzymatic complex belongs to the second class of enzyme, which are the transferases. The complex is formed by 574 amino acid residues, divided into two different proteins nammed as Methyltransferase Like number 3 and 14. [[Image:writers.png]] | This enzymatic complex belongs to the second class of enzyme, which are the transferases. The complex is formed by 574 amino acid residues, divided into two different proteins nammed as Methyltransferase Like number 3 and 14. [[Image:writers.png]] | ||
| Line 44: | Line 44: | ||
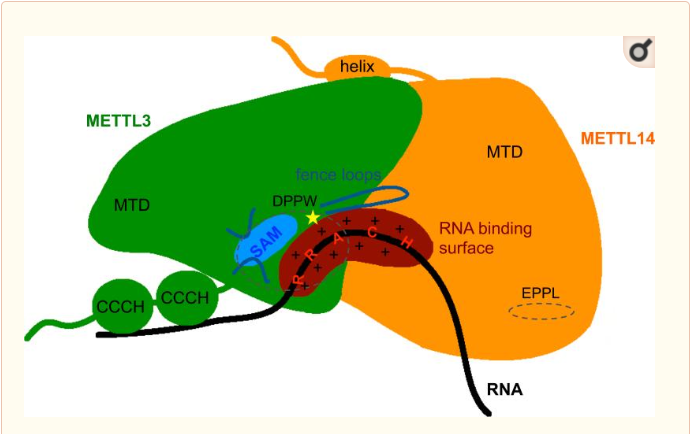
Thus, METTL3 is the catalytic subunit of the complex and METTL14 enhances the methyltransferasese activity by stabilizing the complex structure and binding to messenger RNA by enabling the recognition of its consensus sequence. | Thus, METTL3 is the catalytic subunit of the complex and METTL14 enhances the methyltransferasese activity by stabilizing the complex structure and binding to messenger RNA by enabling the recognition of its consensus sequence. | ||
| - | Both MTD have approximatly 25% sequence homology.Despite some common point the methyltransferase domain of METTL14 has extra terminal extensions, with an unusual N-terminal extension which is approximately 50 amino acid long. In the one hand the N-terminal extension create along helix which go through the domain and allow a close contact with the MTD of the catalytic subunit of the complex. This contact is made by several loops and shorter helical segments. In other hand, the C-terminal helix of the MTD of METTL14 is antiparallel to the N-terminal extension helix in order to stabilize its position. This allow the formation of a broad interdomain binding interface,and also others contact point along the terminal extensions, which are stabilizing the positions of both domains neccesary for the function. | + | Both MTD have approximatly 25% sequence homology.Despite some common point the methyltransferase domain of METTL14 has extra terminal extensions, with an unusual N-terminal extension which is approximately 50 amino acid long. In the one hand the N-terminal extension create along helix which go through the domain and allow a close contact with the MTD of the catalytic subunit of the complex. This contact is made by several loops and shorter helical segments. In other hand, the C-terminal helix of the MTD of METTL14 is antiparallel to the N-terminal extension helix in order to stabilize its position. This allow the formation of a broad interdomain binding interface,and also others contact point along the terminal extensions, which are stabilizing the positions of both domains neccesary for the function.<Structure load='5YZ9' size='350' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' /> |
'''Zinc finger domain of the METTL3 subunit''' | '''Zinc finger domain of the METTL3 subunit''' | ||
| - | + | ||
Zinc finger domain of the METTL3 N6-methyladenosine methyltransferase, is a RNA binding domain of the complex. This perticular domain of the two proteins complex allow the protein to make interactions with the RNA molecule to modify | Zinc finger domain of the METTL3 N6-methyladenosine methyltransferase, is a RNA binding domain of the complex. This perticular domain of the two proteins complex allow the protein to make interactions with the RNA molecule to modify | ||
Revision as of 16:37, 29 December 2018
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
Crystal structure of the catalytic domains of Mettl3/Mettl14 complexInsert caption here
Drag the structure with the mouse to rotate
Insert caption here |
| Drag the structure with the mouse to rotate |
The complex METTL3/METTL14 is a heterodimer enzymatic complex involved into RNA post-transcriptional modifications by humans. This complex is abble to add a methyl group on adenosin of the RNA, by catalyzing a m6(A) modification.The N(6)-methyladenosine (m(6)A) is a quite common, reversible chemical modification of RNAs molecules, which plays a key role in several different biological fonctions. This post-transcriptional modification can be added by WRITERS, recognized by READERS and also removed byr ERASERS. The METTL3/METTL14 complex plays the role of writer.
This enzymatic complex belongs to the second class of enzyme, which are the transferases. The complex is formed by 574 amino acid residues, divided into two different proteins nammed as Methyltransferase Like number 3 and 14. 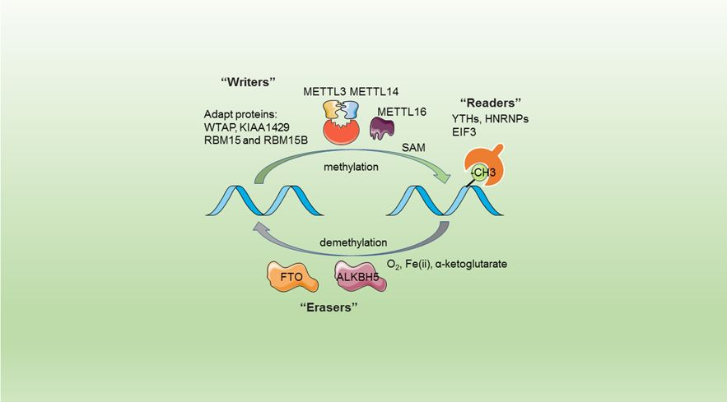
| |||||||||||
References
- ↑ . PMID:216315890657
- ↑ . PMID:216315890657
- ↑ Wang P, Doxtader KA, Nam Y. Structural Basis for Cooperative Function of Mettl3 and Mettl14 Methyltransferases. Mol Cell. 2016 Jul 21;63(2):306-17. doi: 10.1016/j.molcel.2016.05.041. Epub 2016 , Jun 30. PMID:27373337 doi:http://dx.doi.org/10.1016/j.molcel.2016.05.041
- ↑ Wang X, Feng J, Xue Y, Guan Z, Zhang D, Liu Z, Gong Z, Wang Q, Huang J, Tang C, Zou T, Yin P. Structural basis of N(6)-adenosine methylation by the METTL3-METTL14 complex. Nature. 2016 May 25;534(7608):575-8. doi: 10.1038/nature18298. PMID:27281194 doi:http://dx.doi.org/10.1038/nature18298
- ↑ Sledz P, Jinek M. Structural insights into the molecular mechanism of the m(6)A writer complex. Elife. 2016 Sep 14;5. pii: e18434. doi: 10.7554/eLife.18434. PMID:27627798 doi:http://dx.doi.org/10.7554/eLife.18434
- ↑ Wang X, Huang J, Zou T, Yin P. Human m(6)A writers: Two subunits, 2 roles. RNA Biol. 2017 Mar 4;14(3):300-304. doi: 10.1080/15476286.2017.1282025. Epub 2017, Jan 25. PMID:28121234 doi:http://dx.doi.org/10.1080/15476286.2017.1282025
- ↑ doi: https://dx.doi.org/10.2210/pdb5K7M/pdb