Arginine kinase
From Proteopedia
(Difference between revisions)
| Line 13: | Line 13: | ||
== Structural highlights == | == Structural highlights == | ||
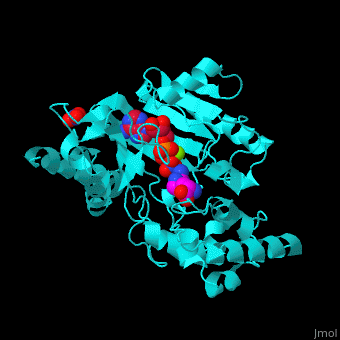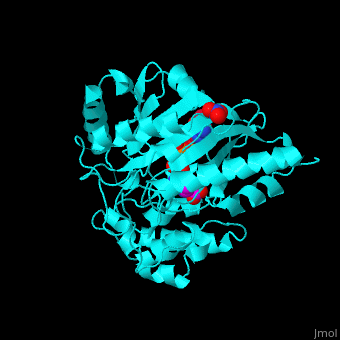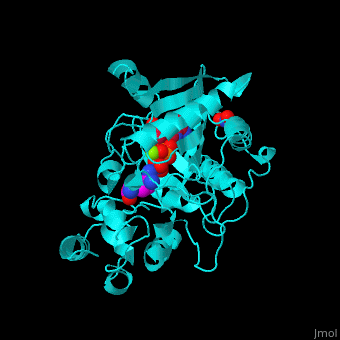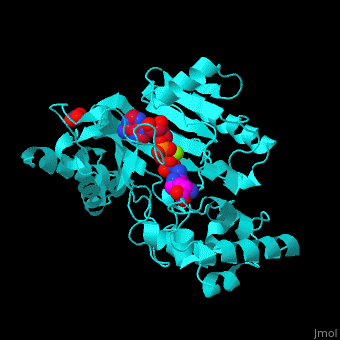
| - | The <scene name='71/715906/Cv/ | + | The <scene name='71/715906/Cv/3'>active site</scene> of AK is located between its N-terminal helical region and the larger C-terminal region. <ref>PMID:9671698</ref> Water molecules are shown as red spheres. <scene name='71/715906/Cv/4'>Mg+2 coordination site</scene>. |
</StructureSection> | </StructureSection> | ||
Revision as of 11:08, 6 January 2019
| |||||||||||
3D structures of arginine kinase
Updated on 06-January-2019
References
- ↑ Wang Z, Qiao Z, Ye S, Zhang R. Structure of a double-domain phosphagen kinase reveals an asymmetric arrangement of the tandem domains. Acta Crystallogr D Biol Crystallogr. 2015 Apr;71(Pt 4):779-89. doi:, 10.1107/S1399004715001169. Epub 2015 Mar 26. PMID:25849389 doi:http://dx.doi.org/10.1107/S1399004715001169
- ↑ Zhou G, Somasundaram T, Blanc E, Parthasarathy G, Ellington WR, Chapman MS. Transition state structure of arginine kinase: implications for catalysis of bimolecular reactions. Proc Natl Acad Sci U S A. 1998 Jul 21;95(15):8449-54. PMID:9671698