We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1480
From Proteopedia
(Difference between revisions)
| Line 12: | Line 12: | ||
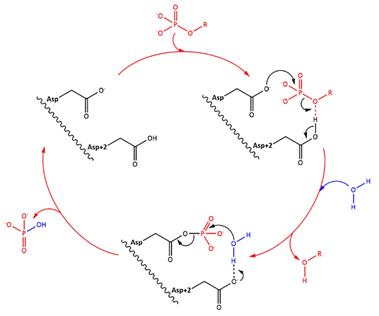
Fig 1. Simplified catalytic mechanism of HAD phosphatases. Asp acts as a nucleophile and Asp+2 as a acid/base. The phosphorylated substrate and the respective leaving groups are shown in red, and the water nucleophile is in blue. (Source: Do metabolic HAD phosphatases moonlight as protein phosphatases?, A. Gohla, 2018). In the human LHPP, the Asp+2 is replaced by a Serine. | Fig 1. Simplified catalytic mechanism of HAD phosphatases. Asp acts as a nucleophile and Asp+2 as a acid/base. The phosphorylated substrate and the respective leaving groups are shown in red, and the water nucleophile is in blue. (Source: Do metabolic HAD phosphatases moonlight as protein phosphatases?, A. Gohla, 2018). In the human LHPP, the Asp+2 is replaced by a Serine. | ||
| + | Expressed in brain (highest expression level in C1 segment of cervical spinal cord), and at lower levels in liver and kidney. | ||
| + | Detected in thyroid at protein level. | ||
== Disease == | == Disease == | ||
Revision as of 13:56, 7 January 2019
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
Structure of the protein LHPP
| |||||||||||