We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1489
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
== '''Function''' == | == '''Function''' == | ||
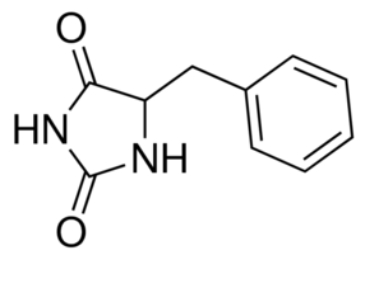
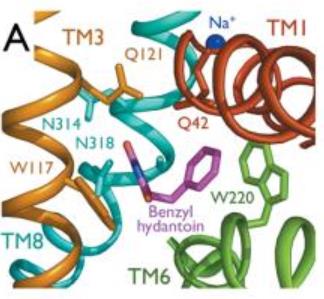
Mhp1 is a transmembrane protein bellowing to the nucleobase-cation-symport-1 (NCS1) transporter family from Microbacterium liquefaciens. It allows the sodium dependent income of indolyl methyl- and benzyl-hydantoins in the cell. Those are part of a salvage metabolic pathway leading to their conversion in amino acids. | Mhp1 is a transmembrane protein bellowing to the nucleobase-cation-symport-1 (NCS1) transporter family from Microbacterium liquefaciens. It allows the sodium dependent income of indolyl methyl- and benzyl-hydantoins in the cell. Those are part of a salvage metabolic pathway leading to their conversion in amino acids. | ||
| + | 2JLN is one of the conformations of Mhp1. It is the outward-facing conformation without substrate. | ||
[[Image:Structure of benzyl-hydantoin.jpg]] | [[Image:Structure of benzyl-hydantoin.jpg]] | ||
| Line 56: | Line 57: | ||
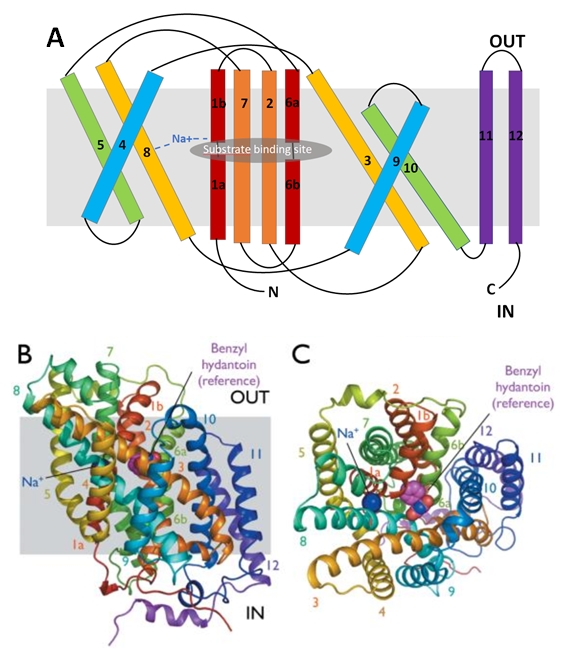
Figure 4: The conformational change upon the substrate binding | Figure 4: The conformational change upon the substrate binding | ||
| + | |||
The binding of the substrate induces conformational changes of the protein allowing it uptake in the cell. | The binding of the substrate induces conformational changes of the protein allowing it uptake in the cell. | ||
| Line 66: | Line 68: | ||
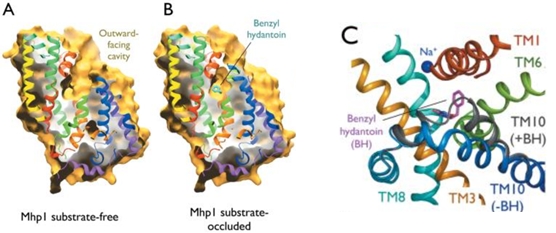
The binding of the substrate in the binding site leads to a switch from the outward-facing open state to the outward-facing occluded state. The TM10 arrangement changes (Figure 4.C) and closes the access to the “OUT” side space of the membrane (Figure 5.A). | The binding of the substrate in the binding site leads to a switch from the outward-facing open state to the outward-facing occluded state. The TM10 arrangement changes (Figure 4.C) and closes the access to the “OUT” side space of the membrane (Figure 5.A). | ||
| - | Then, there is a change from the outward-facing occluded state to the inward-facing occluded state. (Figure 5.B) The substrate-binding site is occluded from the inside of the membrane. It seems that the movement involves the helix bundle of TMs 3 and 8. Moreover, researchers are working on the possibility of a coordinated shifting of TMs 1 and 6 shift with TMs 3 and 8. | + | Then, there is a change from the outward-facing occluded state to the inward-facing occluded state. (Figure 5.B) The substrate-binding site is occluded from the inside of the membrane. It seems that the movement involves the helix bundle of TMs 3 and 8. Moreover, researchers are working on the possibility of a coordinated shifting of TMs 1 and 6 shift with TMs 3 and 8. |
| + | |||
Eventually, there is a switch from the inward-facing occluded state to the inward-facing open state. This allows the release of the substrate in the cytoplasm. However, the structures involved in the change still be unclear (Figure 5.C). | Eventually, there is a switch from the inward-facing occluded state to the inward-facing open state. This allows the release of the substrate in the cytoplasm. However, the structures involved in the change still be unclear (Figure 5.C). | ||
Revision as of 17:54, 9 January 2019
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
2JLN
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644