We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1489
From Proteopedia
(Difference between revisions)
| Line 19: | Line 19: | ||
== '''Structural highlights''' == | == '''Structural highlights''' == | ||
| - | |||
== Global structure == | == Global structure == | ||
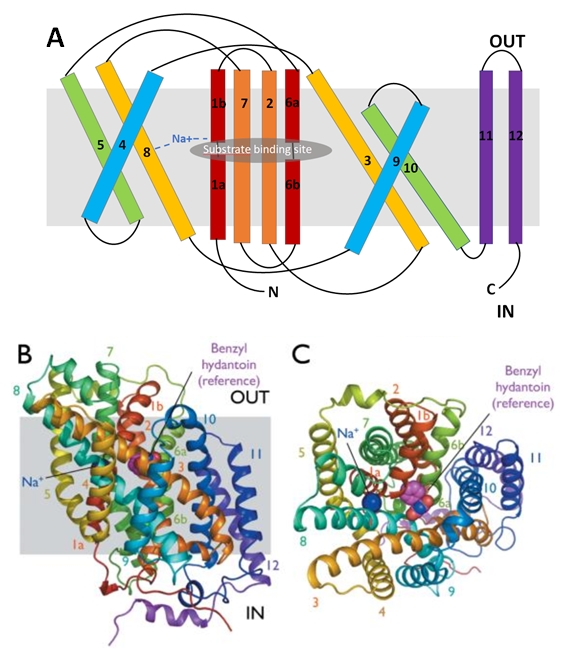
| - | The protein is composed of one chain of 12 transmembrane alpha helices (TMs). They are organised in two repeating units connected by a 59-residue loop (TMs 1-5 and TMs 6-10) and two additional helices (TM 11 and 12). The two repeating units have a symmetrical topology (Figure 2). | + | The protein is composed of one chain of 12 transmembrane alpha helices (TMs). They are organised in two repeating units connected by a 59-residue loop (TMs 1-5 and TMs 6-10) and two additional helices (TM 11 and 12). The two repeating units have a symmetrical topology (''Figure 2''). |
[[Image:Structure of Mhp1 from Microbacterum liquefaciens.jpg]] | [[Image:Structure of Mhp1 from Microbacterum liquefaciens.jpg]] | ||
| - | Figure 2 : Structure of Mhp1 from Microbacterum liquefaciens | + | ''Figure 2 : Structure of Mhp1 from Microbacterum liquefaciens'' |
| - | The central bundle is composed of TMs 1 and 2, twined to the TMs 6 and 7 respectively. In addition, the protein presents a V-shape structure formed by TMs 3 to 5, twined to TMs 8 to 10 (Figure 2.A). | + | The central bundle is composed of TMs 1 and 2, twined to the TMs 6 and 7 respectively. In addition, the protein presents a V-shape structure formed by TMs 3 to 5, twined to TMs 8 to 10 (''Figure 2.A''). |
The substrate- and cation-binding sites are located in the space between the central four-helix-bundle and the outer helix layer. | The substrate- and cation-binding sites are located in the space between the central four-helix-bundle and the outer helix layer. | ||
| Line 38: | Line 37: | ||
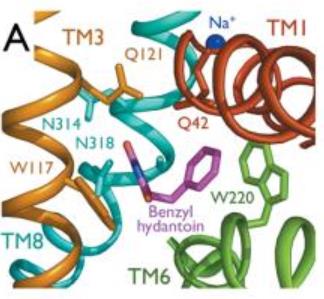
The substrate binding site is located at the break of the TMs 1 and 6. | The substrate binding site is located at the break of the TMs 1 and 6. | ||
| - | The benzyl-hydantoin interacts with the amino acids of the binding site. The hydantoin group establisches pi-stacking interactions with the indole ring of Trp 117 and Trp 220 and hydrogen bounds with Asn 318 and Gln 121. The benzyl ring interacts with Trp 220 and Gln 42 (Figure 3). | + | The benzyl-hydantoin interacts with the amino acids of the binding site. The hydantoin group establisches pi-stacking interactions with the indole ring of Trp 117 and Trp 220 and hydrogen bounds with Asn 318 and Gln 121. The benzyl ring interacts with Trp 220 and Gln 42 (''Figure 3''). |
[[Image:Substrate binding site.jpg]] | [[Image:Substrate binding site.jpg]] | ||
| - | Figure 3 : Substrate binding site | + | ''Figure 3 : Substrate binding site'' |
== Structure of the cation binding site == | == Structure of the cation binding site == | ||
| - | Mhp1 is a sodium dependent protein. The sodium binds at the C-terminal end of TM1a and interacts with TM8 (Figure 2.A). The dipole moment at the C-terminus of TM1a contributes to the binding. | + | Mhp1 is a sodium dependent protein. The sodium binds at the C-terminal end of TM1a and interacts with TM8 (''Figure 2.A''). The dipole moment at the C-terminus of TM1a contributes to the binding. |
Experiments have shown that benzyl-hydantoin increases the affinity of sodium for Mhp1 and reciprocally sodium increases the affinity of benzyl-hydantoin for Mhp1. Therefore, the binding of the substrate and the cation are closely coupled. | Experiments have shown that benzyl-hydantoin increases the affinity of sodium for Mhp1 and reciprocally sodium increases the affinity of benzyl-hydantoin for Mhp1. Therefore, the binding of the substrate and the cation are closely coupled. | ||
| Line 53: | Line 52: | ||
== Conformational states == | == Conformational states == | ||
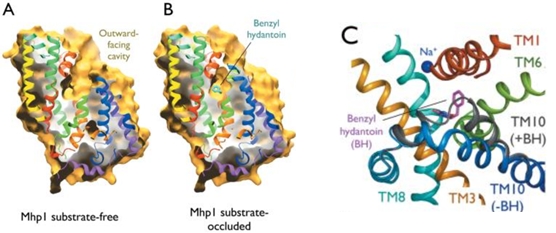
| - | Mhp1 exists in two conformational states depending if the substrate is bound or not: the substrate free structure (-BH), corresponding to the outward-facing open and the substrate bound structure (+BH), corresponding to the outward-facing occluded (Figure 4). | + | Mhp1 exists in two conformational states depending if the substrate is bound or not: the substrate free structure (-BH), corresponding to the outward-facing open and the substrate bound structure (+BH), corresponding to the outward-facing occluded (''Figure 4''). |
[[Image:The conformational change upon the substrate binding.jpg]] | [[Image:The conformational change upon the substrate binding.jpg]] | ||
| - | Figure 4: The conformational change upon the substrate binding | + | ''Figure 4: The conformational change upon the substrate binding'' |
| Line 64: | Line 63: | ||
[[Image:Example.jpg]] | [[Image:Example.jpg]] | ||
| - | Figure 5 : Proposed substrate translocation mechanism by Mhp1 | + | ''Figure 5 : Proposed substrate translocation mechanism by Mhp1'' |
| - | The binding of the substrate in the binding site leads to a switch from the outward-facing open state to the outward-facing occluded state. The TM10 arrangement changes (Figure 4.C) and closes the access to the “OUT” side space of the membrane (Figure 5.A). | + | The binding of the substrate in the binding site leads to a switch from the outward-facing open state to the outward-facing occluded state. The TM10 arrangement changes (''Figure 4.C'') and closes the access to the “OUT” side space of the membrane (''Figure 5.A''). |
| - | Then, there is a change from the outward-facing occluded state to the inward-facing occluded state. (Figure 5.B) The substrate-binding site is occluded from the inside of the membrane. It seems that the movement involves the helix bundle of TMs 3 and 8. Moreover, researchers are working on the possibility of a coordinated shifting of TMs 1 and 6 shift with TMs 3 and 8. | + | Then, there is a change from the outward-facing occluded state to the inward-facing occluded state. (''Figure 5.B'') The substrate-binding site is occluded from the inside of the membrane. It seems that the movement involves the helix bundle of TMs 3 and 8. Moreover, researchers are working on the possibility of a coordinated shifting of TMs 1 and 6 shift with TMs 3 and 8. |
| - | Eventually, there is a switch from the inward-facing occluded state to the inward-facing open state. This allows the release of the substrate in the cytoplasm. However, the structures involved in the change still be unclear (Figure 5.C). | + | Eventually, there is a switch from the inward-facing occluded state to the inward-facing open state. This allows the release of the substrate in the cytoplasm. However, the structures involved in the change still be unclear (''Figure 5.C''). |
Revision as of 18:03, 9 January 2019
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
2JLN
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644