Sandbox Reserved 1480
From Proteopedia
| Line 5: | Line 5: | ||
== Function == | == Function == | ||
The human protein LHPP or phospholysine phosphohistidine inorganic pyrophosphate phosphatase (LHPP) is part of the haloacid dehalogenase (HAD) family. It is a protein of 271 amino acids and is encoded by a seven exon gene positioned on the chromosome 10. | The human protein LHPP or phospholysine phosphohistidine inorganic pyrophosphate phosphatase (LHPP) is part of the haloacid dehalogenase (HAD) family. It is a protein of 271 amino acids and is encoded by a seven exon gene positioned on the chromosome 10. | ||
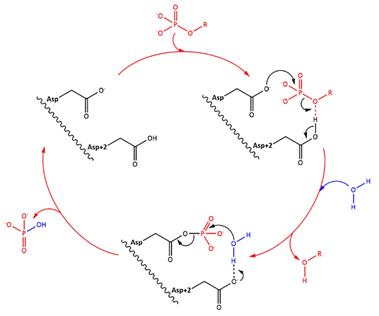
| - | LHPP has two domains, one catalytic domain with a <scene name='80/802654/Mg_ion/1'> magnesium ion</scene> (Mg2+) on the active site and a large C2a-type cap domain, and forms a homodimer in solution. Despite the large cap, the enzyme is not only able to act on small metabolites, but also on phosphoproteins. The protein is found in the nucleus and in the cytoplasm. | + | LHPP has two domains, one catalytic domain with a <scene name='80/802654/Mg_ion/1'> a phosphoric acid and a magnesium ion</scene> (Mg2+) on the <scene name='80/802654/Catalityc_site/1'>active site</scene> and a large C2a-type cap domain, and forms a homodimer in solution. Despite the large cap, the enzyme is not only able to act on small metabolites, but also on phosphoproteins. The protein is found in the nucleus and in the cytoplasm. |
LHPP is a phosphatase with an in vitro activity towards inorganic pyrophosphate, imidodiphosphate, 3‑phosphohistidine and 6‑phospholysine. The enzyme acts more effectively on N-P bonds than O-P bonds. It binds one Mg2+ ion per subunit. | LHPP is a phosphatase with an in vitro activity towards inorganic pyrophosphate, imidodiphosphate, 3‑phosphohistidine and 6‑phospholysine. The enzyme acts more effectively on N-P bonds than O-P bonds. It binds one Mg2+ ion per subunit. | ||
Revision as of 11:51, 10 January 2019
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
Structure of the protein LHPP
| |||||||||||
References
"Molecular cloning of a cDNA for the human phospholysine phosphohistidine inorganic pyrophosphate phosphatase." Yokoi F., Hiraishi H., Izuhara K. J. Biochem. 133:607-614(2003) [PubMed] [Europe PMC] [Abstract]
"The protein histidine phosphatase LHPP is a tumour suppressor." Sravanth K. Hindupur, Marco Colombi, Stephen R. Fuhs, Matthias S. Matter, Yakir Guri, Kevin Adam, Marion Cornu, Salvatore Piscuoglio, Charlotte K. Y. Ng, Charles Betz, Dritan Liko, Luca Quagliata, Suzette Moes, Paul Jenoe, Luigi M. Terracciano, Markus H. Heim, Tony Hunter & Michael N. Hall. (2018) [PubMed] [Main]
"Do metabolic HAD phosphatases moonlight as protein phosphatases?." Antje Gohla. (2018) [BBA - Molecular Cell Research]
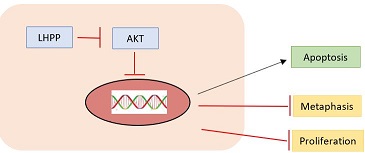
"Down-regulation of LHPP in cervical cancer influences cell proliferation, metastasis and apoptosis by modulating AKT", (2018), [PubMed] [Main]