We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1490
From Proteopedia
(Difference between revisions)
| Line 19: | Line 19: | ||
== Disease == | == Disease == | ||
| - | + | ===• Dominantly inherited venous malformations (VMCM)=== | |
Disease description: A vascular morphogenesis error characterized by dilated serpiginous channels. All mutations occur in the protein kinase domain. | Disease description: A vascular morphogenesis error characterized by dilated serpiginous channels. All mutations occur in the protein kinase domain. | ||
| Line 25: | Line 25: | ||
Arginine at position 849 is found in six residues upstream of the invariant lysine K855 in the kinase domain (sequence preserved among the human, bovine, murine and rat TIE2 sequences). This seems to prove that a basic amino acid is essential for this position. In addition, arginine located a few amino acids before invariant lysine is involved in stabilizing the kinase domain (hydrogen binding of arginine with a proline downstream). It is therefore possible that R849 may also be involved in the stabilization of the kinase domain. Thus, the substitution of R849 by a W could modify the conformation of the kinase domain, leading to a decrease in inhibitory mechanisms and involving autophosphorylation. | Arginine at position 849 is found in six residues upstream of the invariant lysine K855 in the kinase domain (sequence preserved among the human, bovine, murine and rat TIE2 sequences). This seems to prove that a basic amino acid is essential for this position. In addition, arginine located a few amino acids before invariant lysine is involved in stabilizing the kinase domain (hydrogen binding of arginine with a proline downstream). It is therefore possible that R849 may also be involved in the stabilization of the kinase domain. Thus, the substitution of R849 by a W could modify the conformation of the kinase domain, leading to a decrease in inhibitory mechanisms and involving autophosphorylation. | ||
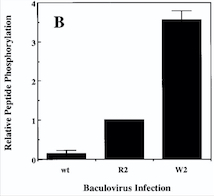
| - | Comparison of the Kinase Activities of Normal and Mutant TIE2 Receptors | + | '''''[[Diagram : Comparison of the Kinase Activities of Normal and Mutant TIE2 Receptors]]''''' |
(B) Cells infected with wild-type baculovirus (wt) or virus expressing normal TIE2 (R2) or mutant TIE2 (W2). Cells expressing the mutation at position 849 (Arginine à Tryptophan) have an autophosphorylation activity 6 to 10 times higher than wild cells. | (B) Cells infected with wild-type baculovirus (wt) or virus expressing normal TIE2 (R2) or mutant TIE2 (W2). Cells expressing the mutation at position 849 (Arginine à Tryptophan) have an autophosphorylation activity 6 to 10 times higher than wild cells. | ||
[[Image:Venous Malformations Diagram.jpg]] | [[Image:Venous Malformations Diagram.jpg]] | ||
| + | With this mutation, Venous Malformations (VMs) contain a Disproportionately high ratio of Endothelial Cells (ECs) to Smooth Muscle Cells (SMCs) | ||
| + | |||
| + | Pictures of immunohistochemistry of VMs with Antibodies against Smooth Muscle Cells 𝛂-Actin. | ||
| + | |||
| + | B = Abnormal channels | ||
| + | C = Normal veins (v) and arteries (a) | ||
| + | Antibodies directed against SMCs 𝛂-Actin from cells with VMs show that the vessels have a specific and abnormal staining (B) compared to normal vessels (C) | ||
| + | Scale bars, 200 𝛍m. | ||
== Relevance == | == Relevance == | ||
Revision as of 15:25, 10 January 2019
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
Kinase Domain of Tyrosine-protein kinase receptor TIE-2 (PDB:6MWE)
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644