Sandbox Reserved 1496
From Proteopedia
| Line 26: | Line 26: | ||
[[Image:Quinucli.jpg|thumb|Structures of BPH-651, a 3-OH quinuclidine inhibitor, bound to CrtM]] | [[Image:Quinucli.jpg|thumb|Structures of BPH-651, a 3-OH quinuclidine inhibitor, bound to CrtM]] | ||
This enzyme consists of 2 sequence-identical polypeptide chains of 287 amino acids<ref>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4866860/]StrainVijay Aswani, Bob Mau, and Sanjay K. Shukla. Complete Genome Sequence of Staphylococcus aureus MCRF184, a Necrotizing Fasciitis-Causing Methicillin-Sensitive Sequence Type 45 Staphylococcus. Genome Announc. 2016 May-Jun; 4(3): e00374-16. | This enzyme consists of 2 sequence-identical polypeptide chains of 287 amino acids<ref>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4866860/]StrainVijay Aswani, Bob Mau, and Sanjay K. Shukla. Complete Genome Sequence of Staphylococcus aureus MCRF184, a Necrotizing Fasciitis-Causing Methicillin-Sensitive Sequence Type 45 Staphylococcus. Genome Announc. 2016 May-Jun; 4(3): e00374-16. | ||
| - | Published online 2016 May 12. doi: 10.1128/genomeA.00374-16 PMCID: PMC4866860 {{PMID|27174283}}</ref>. | + | Published online 2016 May 12. doi: 10.1128/genomeA.00374-16 PMCID: PMC4866860 {{PMID|27174283}}</ref> and is biologically active as a dimer{{cn}}. |
; Ligands | ; Ligands | ||
: 6 Magnesium ions | : 6 Magnesium ions | ||
Revision as of 17:44, 10 January 2019
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
|
Contents |
Presentation of dehydrosqualene synthase
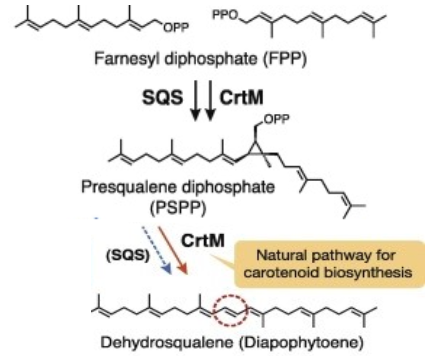
The C30 carotene synthase (also known as 4,4'-diapophytoene (DAP) synthase; dehydrosqualene synthase; CrtM), encoded by crtM gene, is a bacterial carotenoid synthase which is involved in the first step of the pathway that synthesizes staphyloxanthin from farnesyl diphosphate.
This pathway is a subpathway in staphyloxanthin biosynthesis, which is itself part of carotenoid biosynthesis. Carotenoid pathways are branches of the general isoprenoid pathway.
Staphyloxanthin is a carotenoid, which is responsible for the signature golden color of Staphylococcus aureus and also plays the role of virulence factor to human. It has an antioxidant activity that helps the microbe to survive under hight concentration of reactive oxygen species, caused by the host immune system. Having a better comprehension of the synthesis of this carotenoid will help to find a cure to S. aureus related diseases.
Function(Biological reaction)
CrtM catalyses the first step of the synthesis of staphyloxanthin (with a 2-step mechanism)[1]. The gene is located in the staphyloxanthin biosynthesis operon (crtOPQMN), which encodes enzymes to catalyze the synthesis[2]. The product, C(30) carotenoid 4,4'-diapophytoene (dehydrosqualene) is colorless.
- Catalysed reaction (head-to-head condensation)
- 2 (2E,6E)-farnesyl diphosphate (FPP) ↔15-cis-4,4'-diapophytoene + 2 diphosphate
Transferase Activity : Transferring alkyl or aryl groups, other than methyl groups
Metal Ion Binding : requires Mn2+ as cofactor (2 ions per subunit).
Structure
This enzyme consists of 2 sequence-identical polypeptide chains of 287 amino acids[3] and is biologically active as a dimerTemplate:Cn.
- Ligands
- 6 Magnesium ions
- 2 PO42-
- 1 L(+)-tartaric acid
- 2 (3r)-3-biphenyl-4-yl-1-azabicyclo[2.2.2]octan-3-ol
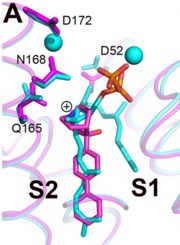
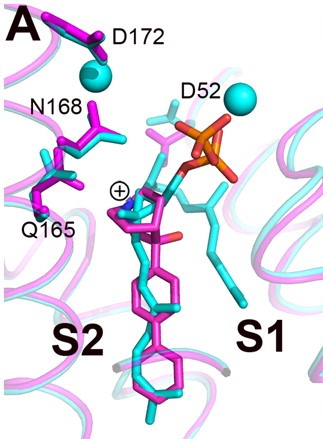
Structures of BPH-651, a 3-OH quinuclidine inhibitor, bound to CrtM
The space group of FsPP·CrtM is P3121, with two molecules per asymmetric unit[4]. Three phosphonosulfonates, which mimic the FPP substract have been identified to have binding/inhibiting activity on CrtM, each with different binding pattern.
Human diseases
The Staphyloxanthin catalyzed by CrtM is a virulence factor linked with infections caused by methicillin-resistant Staphylococcus aureus (MRSA), which is becoming a serious threat[5]. Cells with mutated CrtM are less resistant against oxidative stress, shows lower survival rate and are less pathogenic in mouses[6]. Due to its structural similarity as well as shared inhibitor with human squalene synthase (SQS)[4][7], it is seen as a potential target for virulence factor neutralization therapy, where the idea is to inhibit the pathogen's synthetic pathway. Cationic inhibitors are here of interest as anti-infectives because they can substitute themselves to Mg2+ and inactivate the enzyme.
References
- ↑ [1]Alexandra Clauditz, Alexandra Resch, Karsten-Peter Wieland, Andreas Peschel, and Friedrich Götz. Staphyloxanthin Plays a Role in the Fitness of Staphylococcus aureus and Its Ability To Cope with Oxidative Stress. Infect Immun. 2006 Aug; 74(8): 4950–4953. doi: 10.1128/IAI.00204-06 PMCID: PMC1539600PubMed:16861688
- ↑ [2]Pelz A, Wieland KP, Putzbach K, Hentschel P, Albert K, Götz F. Structure and biosynthesis of staphyloxanthin from Staphylococcus aureus. J Biol Chem. 2005 Sep 16;280(37):32493-8. Epub 2005 Jul 14. PubMed:16020541
- ↑ [3]StrainVijay Aswani, Bob Mau, and Sanjay K. Shukla. Complete Genome Sequence of Staphylococcus aureus MCRF184, a Necrotizing Fasciitis-Causing Methicillin-Sensitive Sequence Type 45 Staphylococcus. Genome Announc. 2016 May-Jun; 4(3): e00374-16. Published online 2016 May 12. doi: 10.1128/genomeA.00374-16 PMCID: PMC4866860 PubMed:27174283
- ↑ 4.0 4.1 [4]Chia-I Liu, George Y. Liu, Yongcheng Song, Fenglin Yin, Mary E. Hensler, Wen-Yin Jeng, Victor Nizet, Andrew H.-J. Wang, and Eric Oldfield. A Cholesterol Biosynthesis Inhibitor Blocks Staphylococcus aureus Virulence. Science. 2008 Mar 7; 319(5868): 1391–1394. Published online 2008 Feb 14. : 10.1126/science.1153018. PMCID: PMC2747771 NIHMSID: NIHMS137229 PubMed:18276850.
- ↑ [5]Klevens RM1, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK; Active Bacterial Core surveillance (ABCs) MRSA Investigators. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007 Oct 17;298(15):1763-71. PubMed:17940231 doi:10.1001/jama.298.15.1763
- ↑ [6]Liu G. Y.; Essex A.; Buchanan J. T.; Datta V.; Hoffman H. M.; Bastian J. F.; Fierer J.; Nizet V. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J. Exp. Med. 2005, 202, 209–215.PMCID: PMC2213009PubMed:16009720.
- ↑ [7]Fu-Yang Lin,Yi-Liang Liu, Kai Li, Rong Cao, Wei Zhu, Jordan Axelson, Ran Pang, and Eric Oldfield. Head-to-Head Prenyl Tranferases: Anti-Infective Drug Targets. J Med Chem. 2012 May 10; 55(9): 4367–4372. Published online 2012 May 1. doi: 10.1021/jm300208p PMCID: PMC3349777 NIHMSID: NIHMS370437 PubMed:22486710