Sandbox Reserved 1489
From Proteopedia
(Difference between revisions)
| Line 26: | Line 26: | ||
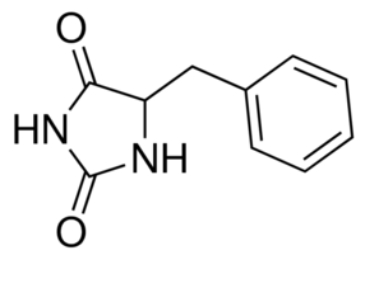
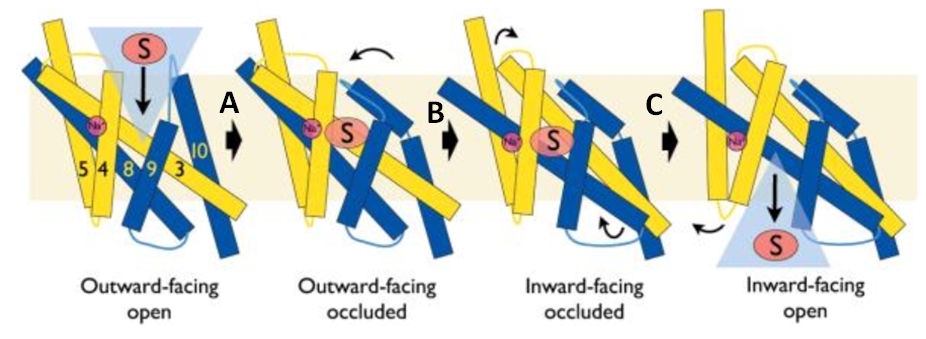
Transporters from the NCS1 family are also important in the toxicity of the antifungal agent, 5‐flucytosine and mutations in the proteins can lead to drug resistance. Mhp1 is an excellent model system for elucidating how substrates or inhibitors, including drugs, are recognised at the molecular level and then taken up into cells by members of the NCS1 transporter family. | Transporters from the NCS1 family are also important in the toxicity of the antifungal agent, 5‐flucytosine and mutations in the proteins can lead to drug resistance. Mhp1 is an excellent model system for elucidating how substrates or inhibitors, including drugs, are recognised at the molecular level and then taken up into cells by members of the NCS1 transporter family. | ||
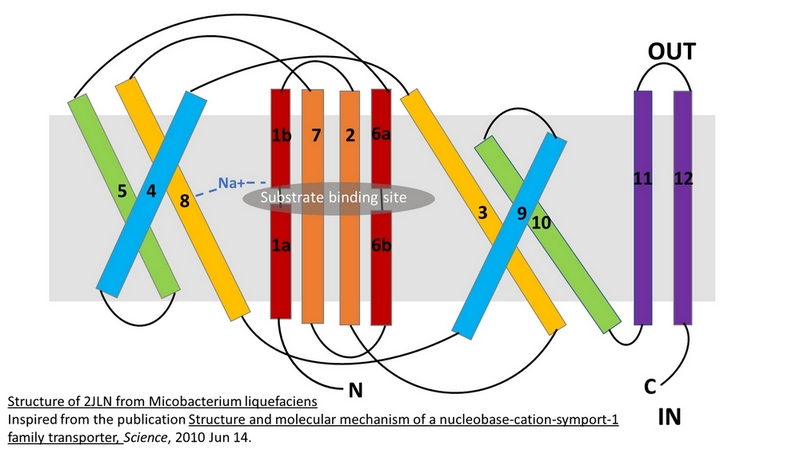
| - | Mhp1 is of more general significance because it is also structurally homologous to other proteins in different subfamilies of the superfamily of secondary transporters. The study of the mechanisms of Mhp1 enables the understanding of other members of this family too. Members of the neurotransmitter‐sodium‐symport family (NSS), solute‐sodium‐symporter family (SSS) and amino acid‐polyamine‐organocation family (APC) are secondary transporters similar to Mhp1. They play important roles in human physiology, being responsible for the accumulation of molecules such as neurotransmitters, sugars, amino acids and drugs into cells.<ref> | + | Mhp1 is of more general significance because it is also structurally homologous to other proteins in different subfamilies of the superfamily of secondary transporters. The study of the mechanisms of Mhp1 enables the understanding of other members of this family too. Members of the neurotransmitter‐sodium‐symport family (NSS), solute‐sodium‐symporter family (SSS) and amino acid‐polyamine‐organocation family (APC) are secondary transporters similar to Mhp1. They play important roles in human physiology, being responsible for the accumulation of molecules such as neurotransmitters, sugars, amino acids and drugs into cells.<ref>PMID 24952894</ref> |
Revision as of 18:32, 10 January 2019
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
2JLN
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
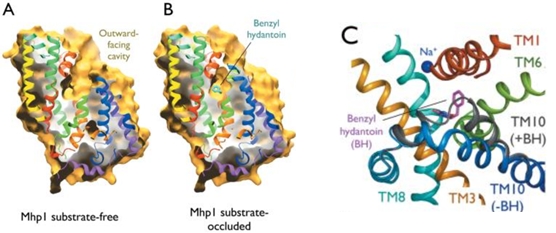
- ↑ Shimamura T, Weyand S, Beckstein O, Rutherford NG, Hadden JM, Sharples D, Sansom MS, Iwata S, Henderson PJ, Cameron AD. Molecular basis of alternating access membrane transport by the sodium-hydantoin transporter Mhp1. Science. 2010 Apr 23;328(5977):470-3. PMID:20413494 doi:328/5977/470
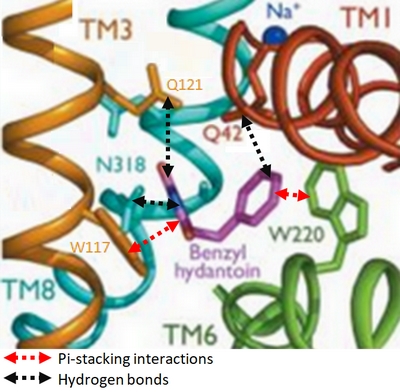
- ↑ Simmons KJ, Jackson SM, Brueckner F, Patching SG, Beckstein O, Ivanova E, Geng T, Weyand S, Drew D, Lanigan J, Sharples DJ, Sansom MS, Iwata S, Fishwick CW, Johnson AP, Cameron AD, Henderson PJ. Molecular mechanism of ligand recognition by membrane transport protein, Mhp1. EMBO J. 2014 Jun 21. pii: e201387557. PMID:24952894 doi:http://dx.doi.org/10.15252/embj.201387557
- ↑ Shimamura T, Weyand S, Beckstein O, Rutherford NG, Hadden JM, Sharples D, Sansom MS, Iwata S, Henderson PJ, Cameron AD. Molecular basis of alternating access membrane transport by the sodium-hydantoin transporter Mhp1. Science. 2010 Apr 23;328(5977):470-3. PMID:20413494 doi:328/5977/470
- ↑ Shimamura T, Weyand S, Beckstein O, Rutherford NG, Hadden JM, Sharples D, Sansom MS, Iwata S, Henderson PJ, Cameron AD. Molecular basis of alternating access membrane transport by the sodium-hydantoin transporter Mhp1. Science. 2010 Apr 23;328(5977):470-3. PMID:20413494 doi:328/5977/470
- ↑ Shimamura T, Weyand S, Beckstein O, Rutherford NG, Hadden JM, Sharples D, Sansom MS, Iwata S, Henderson PJ, Cameron AD. Molecular basis of alternating access membrane transport by the sodium-hydantoin transporter Mhp1. Science. 2010 Apr 23;328(5977):470-3. PMID:20413494 doi:328/5977/470