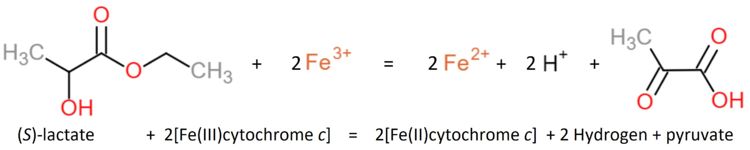We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1501
From Proteopedia
(Difference between revisions)
| Line 10: | Line 10: | ||
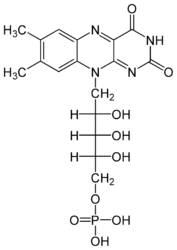
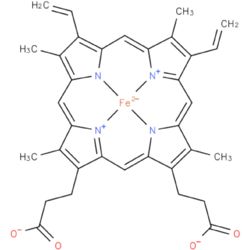
Flavocytochrome b(2) catalyzes dehydrogenation of L-lactate and the coupled cytochrome c reduction. | Flavocytochrome b(2) catalyzes dehydrogenation of L-lactate and the coupled cytochrome c reduction. | ||
| - | Similar oxidants that can be used to perform the reaction ''in vitro'' are ferricyanide, phenazine methosulfate and quinone (experiment first performed 1963 by Nygaard<ref>PMID: 14480786</ref>, later in 1966 described by Symons and Burgoyne<ref> | + | Similar oxidants that can be used to perform the reaction ''in vitro'' are ferricyanide, phenazine methosulfate and quinone (experiment first performed 1963 by Nygaard<ref>PMID: 14480786</ref>, later in 1966 described by Symons and Burgoyne<ref>10.1016/0076-6879(66)09064-5</ref>). |
The yeasts L-lactate dehydrogenase can be inhibited by heavy metals, oxygen, glycerate, oxalate, malate, phenylpyruvate and fatty acids (Nygaard, 1963<ref>PMID: 14480786</ref>). | The yeasts L-lactate dehydrogenase can be inhibited by heavy metals, oxygen, glycerate, oxalate, malate, phenylpyruvate and fatty acids (Nygaard, 1963<ref>PMID: 14480786</ref>). | ||
The enzyme shows a specificity for L-lactate but none for the D-isomer or -hydroxybutyrate. | The enzyme shows a specificity for L-lactate but none for the D-isomer or -hydroxybutyrate. | ||
Revision as of 19:01, 10 January 2019
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
| |||||||||||
References
- ↑ NYGAARD AP. Various forms of D- and L-lactate dehydrogenases in yeast. Ann N Y Acad Sci. 1961 Nov 2;94:774-9. PMID:14480786
- ↑ 10.1016/0076-6879(66)09064-5
- ↑ NYGAARD AP. Various forms of D- and L-lactate dehydrogenases in yeast. Ann N Y Acad Sci. 1961 Nov 2;94:774-9. PMID:14480786
- ↑ 1972PMID: 4336855
- ↑ 1972PMID: 4152980
- ↑ Groudinsky O. Study of heme-protein linkage in cytochrome b2. Destruction of a crucial histidine residue by photooxidation of "apo" cytochrome b2 core in the presence of rose bengal. Eur J Biochem. 1971 Feb;18(4):480-4. PMID:5545004
- ↑ 1972PMID: 4575975
- ↑ APPLEBY CA, MORTON RK. Lactic dehydrogenase and cytochrome b2 of baker's yeast; purification and crystallization. Biochem J. 1959 Mar;71(3):492-9. PMID:13638255
- ↑ Lederer F. On the first steps of lactate oxidation by bakers' yeast L-(plus)-lactate dehydrogenase (cytochrome b2). Eur J Biochem. 1974 Jul 15;46(2):393-9. PMID:4152980
- ↑ Tegoni M, Cambillau C. The 2.6-A refined structure of the Escherichia coli recombinant Saccharomyces cerevisiae flavocytochrome b2-sulfite complex. Protein Sci. 1994 Feb;3(2):303-13. PMID:8003966
- ↑ Tegoni M, Cambillau C. The 2.6-A refined structure of the Escherichia coli recombinant Saccharomyces cerevisiae flavocytochrome b2-sulfite complex. Protein Sci. 1994 Feb;3(2):303-13. PMID:8003966