We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1489
From Proteopedia
(Difference between revisions)
| Line 91: | Line 91: | ||
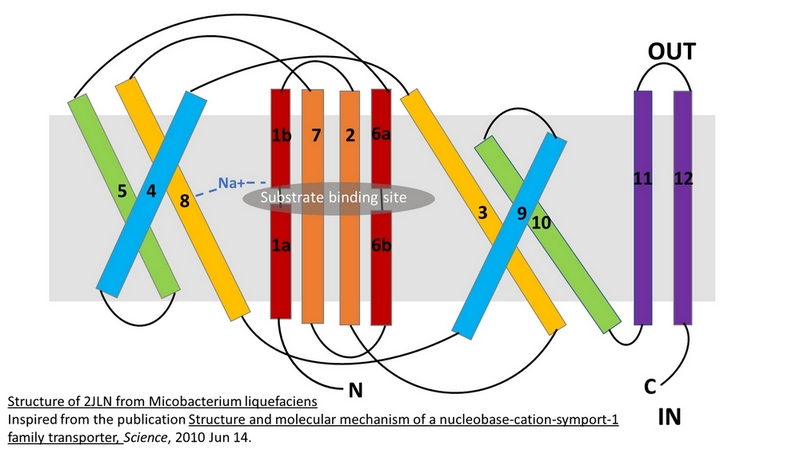
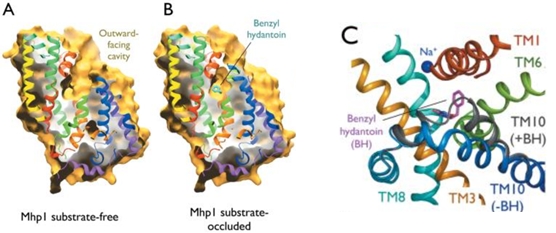
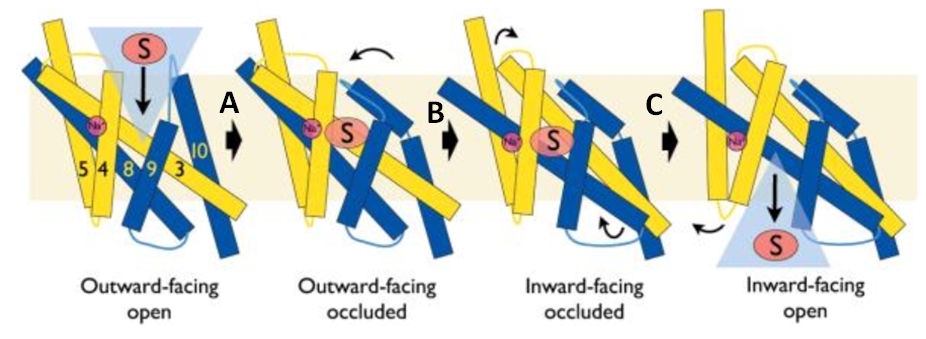
| - | The binding of the substrate in the binding site leads to a switch from the outward-facing open state (2JLN) to the outward-facing occluded state [[4D1B]]. The TM10 arrangement changes (''Figure 4.C'') and closes the access to the “OUT” side space of the membrane (''Figure 5.A''). | + | The binding of the substrate in the binding site leads to a switch from the outward-facing open state (2JLN) to the outward-facing occluded state ([[4D1B]]). The TM10 arrangement changes (''Figure 4.C'') and closes the access to the “OUT” side space of the membrane (''Figure 5.A''). |
| - | Then, there is a change from the outward-facing occluded state to the inward-facing occluded state [[4D1A]]. (''Figure 5.B'') The substrate-binding site is occluded from the inside of the membrane. It seems that the movement involves the helix bundle of TMs 3 and 8. Moreover, researchers are working on the possibility of a coordinated shifting of TMs 1 and 6 shift with TMs 3 and 8. | + | Then, there is a change from the outward-facing occluded state to the inward-facing occluded state ([[4D1A]]). (''Figure 5.B'') The substrate-binding site is occluded from the inside of the membrane. It seems that the movement involves the helix bundle of TMs 3 and 8. Moreover, researchers are working on the possibility of a coordinated shifting of TMs 1 and 6 shift with TMs 3 and 8. |
| - | Eventually, there is a switch from the inward-facing occluded state to the inward-facing open state [[2X79]]. This allows the release of the substrate in the cytoplasm. However, the structures involved in the change still be unclear (''Figure 5.C''). | + | Eventually, there is a switch from the inward-facing occluded state to the inward-facing open state ([[2X79]]). This allows the release of the substrate in the cytoplasm. However, the structures involved in the change still be unclear (''Figure 5.C''). |
Revision as of 19:48, 10 January 2019
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
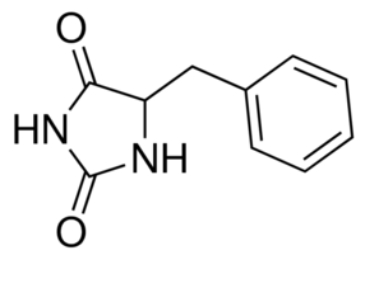
2JLN
| |||||||||||
References
- ↑ Weyand S, Shimamura T, Yajima S, Suzuki S, Mirza O, Krusong K, Carpenter EP, Rutherford NG, Hadden JM, O'Reilly J, Ma P, Saidijam M, Patching SG, Hope RJ, Norbertczak HT, Roach PC, Iwata S, Henderson PJ, Cameron AD. Structure and Molecular Mechanism of a Nucleobase-Cation-Symport-1 Family Transporter. Science. 2008 Oct 16. PMID:18927357
- ↑ Shimamura T, Weyand S, Beckstein O, Rutherford NG, Hadden JM, Sharples D, Sansom MS, Iwata S, Henderson PJ, Cameron AD. Molecular basis of alternating access membrane transport by the sodium-hydantoin transporter Mhp1. Science. 2010 Apr 23;328(5977):470-3. PMID:20413494 doi:328/5977/470
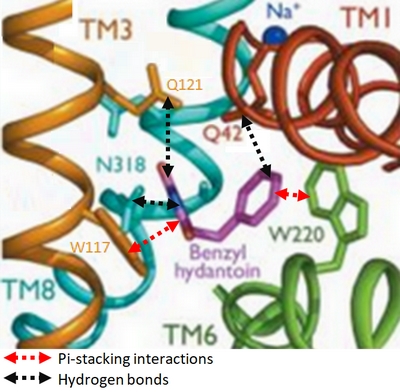
- ↑ Simmons KJ, Jackson SM, Brueckner F, Patching SG, Beckstein O, Ivanova E, Geng T, Weyand S, Drew D, Lanigan J, Sharples DJ, Sansom MS, Iwata S, Fishwick CW, Johnson AP, Cameron AD, Henderson PJ. Molecular mechanism of ligand recognition by membrane transport protein, Mhp1. EMBO J. 2014 Jun 21. pii: e201387557. PMID:24952894 doi:http://dx.doi.org/10.15252/embj.201387557
- ↑ Shimamura T, Weyand S, Beckstein O, Rutherford NG, Hadden JM, Sharples D, Sansom MS, Iwata S, Henderson PJ, Cameron AD. Molecular basis of alternating access membrane transport by the sodium-hydantoin transporter Mhp1. Science. 2010 Apr 23;328(5977):470-3. PMID:20413494 doi:328/5977/470
- ↑ Shimamura T, Weyand S, Beckstein O, Rutherford NG, Hadden JM, Sharples D, Sansom MS, Iwata S, Henderson PJ, Cameron AD. Molecular basis of alternating access membrane transport by the sodium-hydantoin transporter Mhp1. Science. 2010 Apr 23;328(5977):470-3. PMID:20413494 doi:328/5977/470
- ↑ Shimamura T, Weyand S, Beckstein O, Rutherford NG, Hadden JM, Sharples D, Sansom MS, Iwata S, Henderson PJ, Cameron AD. Molecular basis of alternating access membrane transport by the sodium-hydantoin transporter Mhp1. Science. 2010 Apr 23;328(5977):470-3. PMID:20413494 doi:328/5977/470