We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1489
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
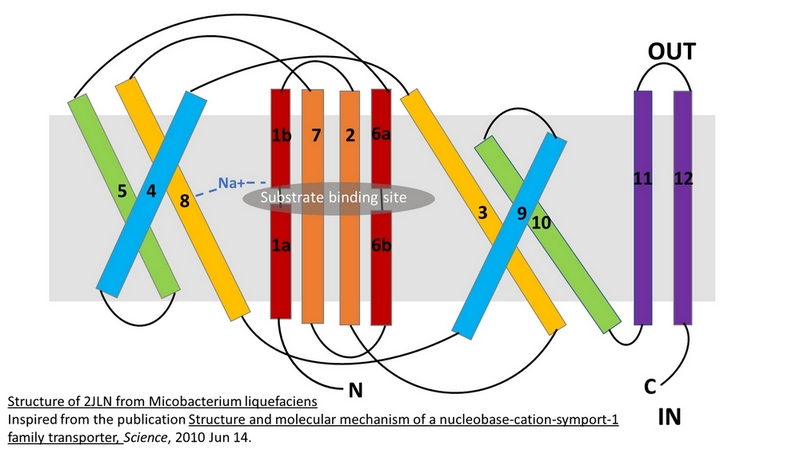
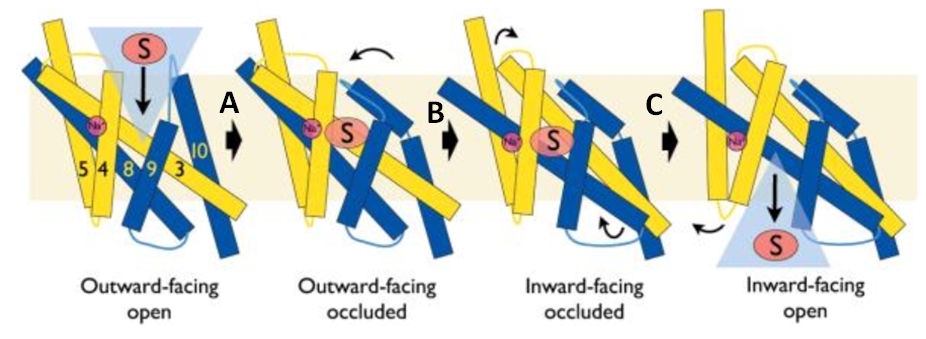
Mhp1 is a trans-membrane protein bellowing to the nucleobase-cation-symport-1 (NCS1) transporter family from Microbacterium liquefaciens. | Mhp1 is a trans-membrane protein bellowing to the nucleobase-cation-symport-1 (NCS1) transporter family from Microbacterium liquefaciens. | ||
| - | A secondary transporter, like Mhp1, effects the cellular uptake and release of a wide range of substances across biological membranes in all organisms. This is done by coupling the uphill movement of the substrate against its concentration gradient with the energetically favorable downhill gradient of a second substrate, often a proton or a cation. The kinetics and thermodynamics of the transporters can be explained by their alternating conformations.<ref>PMID 20413494</ref> | + | A secondary transporter, like Mhp1, effects the cellular uptake and release of a wide range of substances across biological membranes in all organisms. This is done by coupling the uphill movement of the substrate against its concentration gradient with the energetically favorable downhill gradient of a second substrate, often a proton or a cation. The kinetics and thermodynamics of the transporters can be explained by their alternating conformations.<ref name="2">PMID 20413494</ref> |
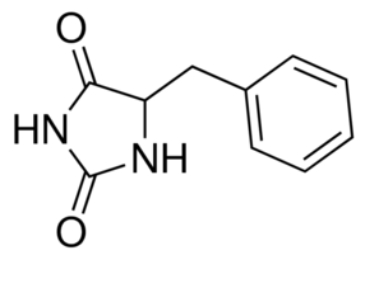
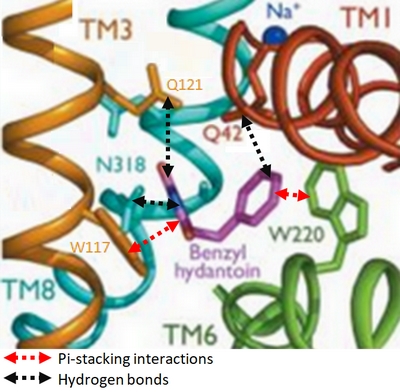
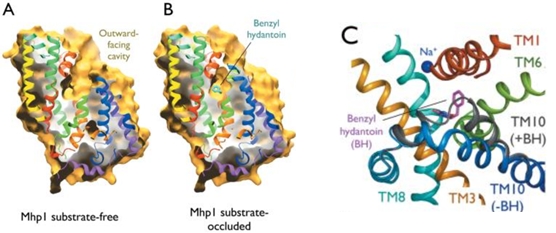
In the case of Mhp1, it allows the sodium dependent income of indolyl methyl- and benzyl-hydantoins (''Figure 1'') in the cell. Those are part of a salvage metabolic pathway leading to their conversion in amino acids. | In the case of Mhp1, it allows the sodium dependent income of indolyl methyl- and benzyl-hydantoins (''Figure 1'') in the cell. Those are part of a salvage metabolic pathway leading to their conversion in amino acids. | ||
| Line 31: | Line 31: | ||
=='''Disease'''== | =='''Disease'''== | ||
| - | Dysfunction of members of the transporters family in humans can lead to diseases including neurological and kidney disorders. Other members are implicated in cancer as they can supply tumor cells with nutrients, cause drug resistance and/or provide a means of treatment.<ref name=" | + | Dysfunction of members of the transporters family in humans can lead to diseases including neurological and kidney disorders. Other members are implicated in cancer as they can supply tumor cells with nutrients, cause drug resistance and/or provide a means of treatment.<ref name="2" /> |
Revision as of 20:17, 10 January 2019
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
2JLN
| |||||||||||
References
- ↑ Weyand S, Shimamura T, Yajima S, Suzuki S, Mirza O, Krusong K, Carpenter EP, Rutherford NG, Hadden JM, O'Reilly J, Ma P, Saidijam M, Patching SG, Hope RJ, Norbertczak HT, Roach PC, Iwata S, Henderson PJ, Cameron AD. Structure and Molecular Mechanism of a Nucleobase-Cation-Symport-1 Family Transporter. Science. 2008 Oct 16. PMID:18927357
- ↑ Simmons KJ, Jackson SM, Brueckner F, Patching SG, Beckstein O, Ivanova E, Geng T, Weyand S, Drew D, Lanigan J, Sharples DJ, Sansom MS, Iwata S, Fishwick CW, Johnson AP, Cameron AD, Henderson PJ. Molecular mechanism of ligand recognition by membrane transport protein, Mhp1. EMBO J. 2014 Jun 21. pii: e201387557. PMID:24952894 doi:http://dx.doi.org/10.15252/embj.201387557
- ↑ Shimamura T, Weyand S, Beckstein O, Rutherford NG, Hadden JM, Sharples D, Sansom MS, Iwata S, Henderson PJ, Cameron AD. Molecular basis of alternating access membrane transport by the sodium-hydantoin transporter Mhp1. Science. 2010 Apr 23;328(5977):470-3. PMID:20413494 doi:328/5977/470
- ↑ Shimamura T, Weyand S, Beckstein O, Rutherford NG, Hadden JM, Sharples D, Sansom MS, Iwata S, Henderson PJ, Cameron AD. Molecular basis of alternating access membrane transport by the sodium-hydantoin transporter Mhp1. Science. 2010 Apr 23;328(5977):470-3. PMID:20413494 doi:328/5977/470