This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 1493
From Proteopedia
| Line 9: | Line 9: | ||
<StructureSection load='2vdl' size='340' side='right' caption='2VDL Headpiece of integrin αIIbβ3' scene=''> | <StructureSection load='2vdl' size='340' side='right' caption='2VDL Headpiece of integrin αIIbβ3' scene=''> | ||
| - | This is a default text for your page ''''''. Click above on '''edit this page''' to modify. Be careful with the < and > signs. | ||
| - | You may include any references to papers as in: the use of JSmol in Proteopedia <ref>DOI 10.1002/ijch.201300024</ref> or to the article describing Jmol <ref>PMID:21638687</ref> to the rescue. | ||
== Structure == | == Structure == | ||
| Line 24: | Line 22: | ||
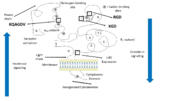
The αIIb subunit (glycoprotein IIb) is composed of 1008 amino acids unevenly distributed over 2 chains. A part forms a light chain which comports a cytoplasmic tail of 20 amino acids and a transmembrane helix. An extracellular disulfide segment links this chain to the heavy extracellular domain which is part of the headpiece of the integrin. | The αIIb subunit (glycoprotein IIb) is composed of 1008 amino acids unevenly distributed over 2 chains. A part forms a light chain which comports a cytoplasmic tail of 20 amino acids and a transmembrane helix. An extracellular disulfide segment links this chain to the heavy extracellular domain which is part of the headpiece of the integrin. | ||
| - | The extracellular N-Terminus forms a cap over the β-propeller domain which is folded by seven successive blades of aminoterminal repeats. Each blade is composed of 4 antiparallel β strands located in each repeat and connected by loops | + | The extracellular N-Terminus forms a cap over the β-propeller domain which is folded by seven successive blades of aminoterminal repeats. Each blade is a β-hairpin loop-like structure composed of 4 antiparallel β strands located in each repeat and connected by loops of the surface. This β-propeller is linked to a thigh and two calf domains, which form the leg structure that supports the heavy head. The total forms the stalk of the αIIb subunit. The region between the thigh and the first calf domain is the site at which the head region bends (in the inactivated form of the integrin) is called the knee of the subunit. |
| - | The β-propeller hosts multiple cation biding sites. The last 3 or 4 blades bind Ca2+ ions which influence ligand binding on the lower side of the blades. The I domain inserted between blades 2 and 3 in the β-propeller follows a Rossman fold with five β-sheets surrounded by seven α-helices. Ligand binding occurs between the β-propeller and the β I domain of the β3 subunit via a coordinating Mg2+ ion in the MIDAS of the β3 subunit. | + | The β-propeller hosts multiple cation biding sites. The last 3 or 4 blades bind Ca2+ ions which influence ligand binding on the lower side of the blades and plays an important role in biogenesis and stability of the heterodimer. The I domain inserted between blades 2 and 3 in the β-propeller follows a Rossman fold with five β-sheets surrounded by seven α-helices. Ligand binding occurs between the β-propeller and the β I domain of the β3 subunit via a coordinating Mg2+ ion in the MIDAS of the β3 subunit. |
The RGD binding site (Arg-Gly-Asp) is in a crevice in this region, inserted between the β-propeller and β I domains. The Arg side chain is located in a groove on the upper surface of the propeller and the Asp carboxylate protruding into a cleft on the β I surface. | The RGD binding site (Arg-Gly-Asp) is in a crevice in this region, inserted between the β-propeller and β I domains. The Arg side chain is located in a groove on the upper surface of the propeller and the Asp carboxylate protruding into a cleft on the β I surface. | ||
| Line 65: | Line 63: | ||
αIIbβ3 can also recognize and bind to the Arg-Gly-Asp (RGD) sequence. This pattern is present within flexible loop regions of fibrinogen α-subunit as well as in other ligands. Indeed, fibronectin binds to αIIbβ3 thanks an Arg-Gly-Asp-Ser-Pro sequence (which is located at the apex of a flexible loop between two β-strands) and von Willebrand factor thanks to the Arg-Gly-Asp sequence in the C1 domain. | αIIbβ3 can also recognize and bind to the Arg-Gly-Asp (RGD) sequence. This pattern is present within flexible loop regions of fibrinogen α-subunit as well as in other ligands. Indeed, fibronectin binds to αIIbβ3 thanks an Arg-Gly-Asp-Ser-Pro sequence (which is located at the apex of a flexible loop between two β-strands) and von Willebrand factor thanks to the Arg-Gly-Asp sequence in the C1 domain. | ||
| - | RGD binding present similar features to binding of the γC domain of fibrinogen to the KQAGDV binding site: the Asp side chain coordinates the Mg2+ ion in MIDAS | + | RGD binding present similar features to binding of the γC domain of fibrinogen to the KQAGDV binding site: the Gly residue is in the same pocket between the two subunits, and the Asp side chain coordinates the Mg2+ ion in MIDAS. Then the Asp side chain forms hydrogen bonds toamide groups including two in the β I domain. The side chain of Arg (along the same pocket as the Lys side chain of the KQAGDV motif of γC) enables to position its guanidinium group for hydrogen bonding to Asp 224. |
Structural data revealed the HHLGGAKQAGDV peptide of the γC domain of fibrinogen binds to an extended site on the receptor that includes the RGD binding site. There is thus competing between he HHLGGAKQAGDV peptide and RGD patterns. | Structural data revealed the HHLGGAKQAGDV peptide of the γC domain of fibrinogen binds to an extended site on the receptor that includes the RGD binding site. There is thus competing between he HHLGGAKQAGDV peptide and RGD patterns. | ||
| Line 79: | Line 77: | ||
==== The resting state ==== | ==== The resting state ==== | ||
| + | |||
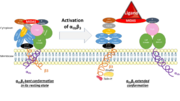
On circulating platelets, integrin αIIbβ3 is maintained in a resting state by intramolecular interactions of the transmembrane and the cytoplasmic domains of its subunits. The transmembrane helices of αIIb and β3 form a complex specifically packed with an inclined geometry. The membrane-proximal regions of the cytoplasmic tails interact thanks to a salt bridge between αIIb-Arg995 and β3-Asp723. The low-affinity compact bent conformation of the inactivated protein allows the platelet to circulate in blood without clotting. | On circulating platelets, integrin αIIbβ3 is maintained in a resting state by intramolecular interactions of the transmembrane and the cytoplasmic domains of its subunits. The transmembrane helices of αIIb and β3 form a complex specifically packed with an inclined geometry. The membrane-proximal regions of the cytoplasmic tails interact thanks to a salt bridge between αIIb-Arg995 and β3-Asp723. The low-affinity compact bent conformation of the inactivated protein allows the platelet to circulate in blood without clotting. | ||
==== Initiation of activation ==== | ==== Initiation of activation ==== | ||
| + | |||
Affinity modulation is initiated at the cytoplasmic tails. It is induced by various agonists (such as ADP, thrombin, collagen, and epinephrine) which constitute intracellular signals for the binding of cytoskeletal proteins (talin and kindlin-3) to the cytoplasmic tail of the β3 subunit. For instance, once unmasked, the PTB domain of talin binds the NPLY motif of the β3 subunit, which breaks the salt bridge linking the cytoplasmic tails together. The result is the dissociation of the transmembrane α-helices of the two subunits. | Affinity modulation is initiated at the cytoplasmic tails. It is induced by various agonists (such as ADP, thrombin, collagen, and epinephrine) which constitute intracellular signals for the binding of cytoskeletal proteins (talin and kindlin-3) to the cytoplasmic tail of the β3 subunit. For instance, once unmasked, the PTB domain of talin binds the NPLY motif of the β3 subunit, which breaks the salt bridge linking the cytoplasmic tails together. The result is the dissociation of the transmembrane α-helices of the two subunits. | ||
==== Propagation of activation ==== | ==== Propagation of activation ==== | ||
| - | It opens a hinge in the integrin which triggers a very quick succession of subunit shifts that are transmitted from the tail to the extracellular headpiece across the transmembrane domain (inside-out signaling). Movements of helices and loops moves the headpiece to an extended conformation which uncovers the interface between the two subunits countaining ligand binding sites. Integrin is at an intermediate affinity state and can bind ligands. | + | |
| + | It opens a hinge in the integrin which triggers a very quick succession of subunit shifts that are transmitted from the tail to the extracellular headpiece across the transmembrane domain (inside-out signaling). Movements of helices and loops moves the headpiece to an extended conformation which uncovers the interface between the two subunits countaining ligand binding sites. Integrin is at an intermediate affinity state (extended conformation, closed headpiece) and can bind ligands. | ||
[[Image:Activation.png|thumb|right|Activation of the binding site at intermediate affinity]] | [[Image:Activation.png|thumb|right|Activation of the binding site at intermediate affinity]] | ||
| - | The precise mechanisms of activation which | + | The precise mechanisms of activation which occur in the extracellular part of the integrin remain a mystery. Still, the conformational difference of a disulfide-bonded knot localised in the cysteine-rich core of the β3 subunit between inactivation and activation suggests that this region plays a part in activation. It is thought that the cysteine core of the β3 subunit linked to its N-terminal extremity apply a conformational constraint on the ligand binding site. It includes a few cysteines that remain unpaired and which redox state influence the activation of the integrin, which supposed this region could host a redox site. |
==== Activated headpiece ==== | ==== Activated headpiece ==== | ||
| - | Once the ligand binding site has been activated, ligand binding can gradually occur. A swing out of the hybrid domain of the β3 subunit around the active site moves the β3 transmembrane domain away from the αIIb transmembrane domain. As a result, the integrin is switched to an open conformation which facilitates binding to ligands. The active site is at its higher affinity state. | + | |
| + | Once the ligand binding site has been activated, ligand binding can gradually occur. A swing out of the hybrid domain of the β3 subunit around the active site moves the β3 transmembrane domain away from the αIIb transmembrane domain. As a result, the integrin headpiece is switched to an open conformation which facilitates binding to ligands. The active site is at its higher affinity state. | ||
The initial contact with the ligand is reversible, but then irreversible binding prevents ligand from dissociating. Binding also changes the conformation of the ligand and may unmask new binding regions on it. | The initial contact with the ligand is reversible, but then irreversible binding prevents ligand from dissociating. Binding also changes the conformation of the ligand and may unmask new binding regions on it. | ||
Revision as of 01:06, 11 January 2019
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
Integrin αIIbβ3 (2VDL)
Integrin αIIbβ3 (or glycoprotein IIb/IIIa) is a complex present on the membrane of platelets that intervenes in the activation, adherence and aggregation of platelets during clotting. It is a cation-dependant heterodimeric transmembrane receptor containing a large extracellular headpiece and short intracellular tails. It is synthesized in megakaryocytes.
Its particular shape and localisation on the membrane allows both ligand binding and transduction of the activation signal. It is the dominant integrin on platelets with 70,000 to 90,000 receptors expressed on each platelet in the resting state.
The headpiece (2VDL) of integrin αIIbβ3 enables cation-facilitated ligand binding with multiple ligands (most known being fibrinogen, fibronectin, von Willebrand factors, thrombospondin and vitronectin). Binding affinity is dynamic and depends on the conformational status of the receptor.
| |||||||||||