This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 1493
From Proteopedia
| Line 20: | Line 20: | ||
=== αIIb subunit === | === αIIb subunit === | ||
| - | The αIIb subunit (glycoprotein IIb) is composed of 1008 amino acids unevenly distributed over 2 chains. A part forms a light chain which comports a cytoplasmic tail of 20 amino acids and a transmembrane helix. An extracellular disulfide segment links this chain to the heavy extracellular domain which is part of the headpiece of the integrin. | + | The αIIb subunit (glycoprotein IIb) is composed of 1008 amino acids unevenly distributed over 2 chains. A part forms a light chain which comports a cytoplasmic tail of 20 amino acids and a transmembrane helix. An extracellular disulfide segment links this chain to the <scene name='80/802667/Alpha_head/1'>heavy extracellular domain</scene> which is part of the headpiece of the integrin. |
The extracellular N-Terminus forms a cap over the β-propeller domain which is folded by seven successive blades of aminoterminal repeats. Each blade is a β-hairpin loop-like structure composed of 4 antiparallel β strands located in each repeat and connected by loops of the surface. This β-propeller is linked to a thigh and two calf domains, which form the leg structure that supports the heavy head. The total forms the stalk of the αIIb subunit. The region between the thigh and the first calf domain is the site at which the head region bends (in the inactivated form of the integrin) is called the knee of the subunit. | The extracellular N-Terminus forms a cap over the β-propeller domain which is folded by seven successive blades of aminoterminal repeats. Each blade is a β-hairpin loop-like structure composed of 4 antiparallel β strands located in each repeat and connected by loops of the surface. This β-propeller is linked to a thigh and two calf domains, which form the leg structure that supports the heavy head. The total forms the stalk of the αIIb subunit. The region between the thigh and the first calf domain is the site at which the head region bends (in the inactivated form of the integrin) is called the knee of the subunit. | ||
| Line 32: | Line 32: | ||
The β3 subunit (glycoprotein IIIa) is a 762 amino acids polypeptide chain. | The β3 subunit (glycoprotein IIIa) is a 762 amino acids polypeptide chain. | ||
| - | Its head is composed of a β I domain which has a fold similar to the I domains of the head of the α subunit. It has a Mg2+ coordinating metal ion dependent adhesion site (MIDAS) motif and a site adjacent to MIDAS (ADMIDAS) which coordinate ions. | + | Its <scene name='80/802667/Beta_head/1'>head</scene> is composed of a β I domain which has a fold similar to the I domains of the head of the α subunit. It has a <scene name='80/802667/Mg_in_beta_head_midas/1'>Mg2+</scene> coordinating metal ion dependent adhesion site (MIDAS) motif and a site adjacent to MIDAS (ADMIDAS) which coordinate ions. |
Its stalk is mainly composed of a plexin-sempahorin-integrin (PSI) domain and | Its stalk is mainly composed of a plexin-sempahorin-integrin (PSI) domain and | ||
| Line 51: | Line 51: | ||
Multiple cation binding sites of integrin are located in the head of both subunits and are indirectly involved in ligand binding. Calcium ion in the β-propeller of αIIb coordinate EF-hand patterns (helix-loop-helix calcium binding patterns) which contribute to the structure of the active site. | Multiple cation binding sites of integrin are located in the head of both subunits and are indirectly involved in ligand binding. Calcium ion in the β-propeller of αIIb coordinate EF-hand patterns (helix-loop-helix calcium binding patterns) which contribute to the structure of the active site. | ||
| - | The β I domain which also interacts with the ligand includes 3 metal ion binding sites: a Mg2+ ion in MIDAS surrounded by 2 Ca2+ ions (including one from AMIDAS). MIDAS Mg2+ ion coordinates the Asp side chain of ligands containing RGD. | + | The β I domain which also interacts with the ligand includes 3 metal ion binding sites: a <scene name='80/802667/Mg_in_beta_head_midas/1'>Mg2+</scene> ion in MIDAS surrounded by 2 Ca2+ ions (including one from AMIDAS). MIDAS <scene name='80/802667/Mg_in_beta_head_midas/1'>Mg2+</scene> ion coordinates the Asp side chain of ligands containing RGD. |
AMIDAS binds an inhibitory Ca2+ ion and an activating Mn2+ ion resulting in conformational changes. It shows the importance of cation binding sites in activity modulation. | AMIDAS binds an inhibitory Ca2+ ion and an activating Mn2+ ion resulting in conformational changes. It shows the importance of cation binding sites in activity modulation. | ||
| Line 57: | Line 57: | ||
=== KQAGDV binding site === | === KQAGDV binding site === | ||
| - | On stimulated platelets, αIIbβ3 has a highly specific receptor for the plasma protein fibrinogen. The KQAGDV binding site interacts with the fibrinogen γ-chain C terminus at the γHHLGGAKQAGDV sequence (residues 400 to 411 of γC). KQAGD is the minimal binding motif for αIIbβ3. The Asp side chain interacts with Mg2+ (MIDAS) and the Gly residue is in a pocket between the two subunits. The peptide ligand interacts with an extended binding site on the integrin and has a turn in the backbone that leads the Lys side chain into a pocket, so that its ammonium group is involved in hydrogen bonding with the αIIb subunit. | + | On stimulated platelets, αIIbβ3 has a highly specific receptor for the plasma protein fibrinogen. The KQAGDV binding site interacts with the fibrinogen γ-chain C terminus at the γHHLGGAKQAGDV sequence (residues 400 to 411 of γC). KQAGD is the minimal binding motif for αIIbβ3. The Asp side chain interacts with <scene name='80/802667/Mg_in_beta_head_midas/1'>Mg2+</scene> (MIDAS) and the Gly residue is in a pocket between the two subunits. The peptide ligand interacts with an extended binding site on the integrin and has a turn in the backbone that leads the Lys side chain into a pocket, so that its ammonium group is involved in hydrogen bonding with the αIIb subunit. |
=== RGD binding site === | === RGD binding site === | ||
| Line 63: | Line 63: | ||
αIIbβ3 can also recognize and bind to the Arg-Gly-Asp (RGD) sequence. This pattern is present within flexible loop regions of fibrinogen α-subunit as well as in other ligands. Indeed, fibronectin binds to αIIbβ3 thanks an Arg-Gly-Asp-Ser-Pro sequence (which is located at the apex of a flexible loop between two β-strands) and von Willebrand factor thanks to the Arg-Gly-Asp sequence in the C1 domain. | αIIbβ3 can also recognize and bind to the Arg-Gly-Asp (RGD) sequence. This pattern is present within flexible loop regions of fibrinogen α-subunit as well as in other ligands. Indeed, fibronectin binds to αIIbβ3 thanks an Arg-Gly-Asp-Ser-Pro sequence (which is located at the apex of a flexible loop between two β-strands) and von Willebrand factor thanks to the Arg-Gly-Asp sequence in the C1 domain. | ||
| - | RGD binding present similar features to binding of the γC domain of fibrinogen to the KQAGDV binding site: the Gly residue is in the same pocket between the two subunits, and the Asp side chain coordinates the Mg2+ ion in MIDAS. Then the Asp side chain forms hydrogen bonds toamide groups including two in the β I domain. The side chain of Arg (along the same pocket as the Lys side chain of the KQAGDV motif of γC) enables to position its guanidinium group for hydrogen bonding to Asp 224. | + | RGD binding present similar features to binding of the γC domain of fibrinogen to the KQAGDV binding site: the Gly residue is in the same pocket between the two subunits, and the Asp side chain coordinates the <scene name='80/802667/Mg_in_beta_head_midas/1'>Mg2+ ion</scene> in MIDAS. Then the Asp side chain forms hydrogen bonds toamide groups including two in the β I domain. The side chain of Arg (along the same pocket as the Lys side chain of the KQAGDV motif of γC) enables to position its guanidinium group for hydrogen bonding to Asp 224. |
Structural data revealed the HHLGGAKQAGDV peptide of the γC domain of fibrinogen binds to an extended site on the receptor that includes the RGD binding site. There is thus competing between he HHLGGAKQAGDV peptide and RGD patterns. | Structural data revealed the HHLGGAKQAGDV peptide of the γC domain of fibrinogen binds to an extended site on the receptor that includes the RGD binding site. There is thus competing between he HHLGGAKQAGDV peptide and RGD patterns. | ||
Revision as of 02:47, 11 January 2019
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
Integrin αIIbβ3 (2VDL)
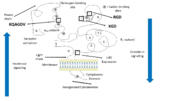
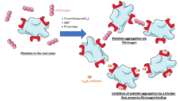
Integrin αIIbβ3 (or glycoprotein IIb/IIIa) is a complex present on the membrane of platelets that intervenes in the activation, adherence and aggregation of platelets during clotting. It is a cation-dependant heterodimeric transmembrane receptor containing a large extracellular headpiece and short intracellular tails. It is synthesized in megakaryocytes.
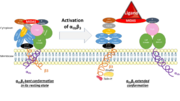
Its particular shape and localisation on the membrane allows both ligand binding and transduction of the activation signal. It is the dominant integrin on platelets with 70,000 to 90,000 receptors expressed on each platelet in the resting state.
The headpiece (2VDL) of integrin αIIbβ3 enables cation-facilitated ligand binding with multiple ligands (most known being fibrinogen, fibronectin, von Willebrand factors, thrombospondin and vitronectin). Binding affinity is dynamic and depends on the conformational status of the receptor.
| |||||||||||