We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1507
From Proteopedia
(Difference between revisions)
| Line 44: | Line 44: | ||
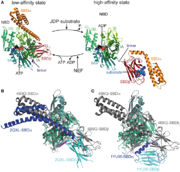
In the activity cycle of Hsp70, unfolded protein are recruited by Hsp70 with the help of J-domain proteins. J-domain proteins binding triggers the ATP hydrolysis in Hsp70 protein. The transformation of ATP into ADP leads to important conformational changes : the PDB domain of Hsp70 adopts a close conformation and binds tightly to the substrate. It’s at this step that intervene Sse1ps. They interact both with Hsp70 and the unfolded protein. The interaction of both the unfolded protein and the chaperone might promote the formation of the complex. | In the activity cycle of Hsp70, unfolded protein are recruited by Hsp70 with the help of J-domain proteins. J-domain proteins binding triggers the ATP hydrolysis in Hsp70 protein. The transformation of ATP into ADP leads to important conformational changes : the PDB domain of Hsp70 adopts a close conformation and binds tightly to the substrate. It’s at this step that intervene Sse1ps. They interact both with Hsp70 and the unfolded protein. The interaction of both the unfolded protein and the chaperone might promote the formation of the complex. | ||
| - | The Hsp70’s PDB which is tightly linked with Sse1p ATP-bound NBD is considered to be the part of the protein helping in the remodelling of misfolded proteins. During this step Hsp70 ADP is liberated. It is the formation of a new bond between Hsp70 and an other ATP that may trigger the dissociation of the complex and the partial or complete folding of the protein. If the protein has not been completely folded, it can bind again to Hsp70 for a new folding cycle<ref> doi: 10.1038/sj.emboj.7601138 </ref>. | + | The Hsp70’s PDB which is tightly linked with Sse1p ATP-bound NBD is considered to be the part of the protein helping in the remodelling of misfolded proteins. During this step Hsp70 ADP is liberated. It is the formation of a new bond between Hsp70 and an other ATP that may trigger the dissociation of the complex and the partial or complete folding of the protein. If the protein has not been completely folded, it can bind again to Hsp70 for a new folding cycle <ref> doi:10.1038/ sj.emboj.7601138 </ref>. |
| Line 55: | Line 55: | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
| - | [PubMed Abstract] | ||
| - | Ballinger, C. A., Connell, P., Wu, Y., Hu, Z., Thompson, L. J., Yin, L. Y., et al. (1999). Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol. Cell. Biol. 19, 4535–4545. | ||
Revision as of 12:52, 11 January 2019
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
3D2F, Crystal structure of a complex of Sse1p and Hsp70
| |||||||||||