We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1490
From Proteopedia
(Difference between revisions)
| Line 17: | Line 17: | ||
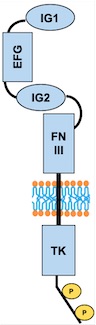
TIE2 maintains the vascular integrity of mature vessels by enhancing endothelial barrier function and inhibiting apoptosis of endothelial cells.<ref>PMID:18425120</ref> | TIE2 maintains the vascular integrity of mature vessels by enhancing endothelial barrier function and inhibiting apoptosis of endothelial cells.<ref>PMID:18425120</ref> | ||
| - | ANGPT1 is a TIE2 agonist : in vitro, it binds to TIE2 and induces its activation via tyrosine phosphorylation. In vivo, it was proven that inactivation of ANGPT1 or over expression of ANGPT2 produce similar effects.<ref name=" | + | ANGPT1 is a TIE2 agonist : in vitro, it binds to TIE2 and induces its activation via tyrosine phosphorylation. In vivo, it was proven that inactivation of ANGPT1 or over expression of ANGPT2 produce similar effects.<ref name="Angiopoietin 2">PMID: 19223473</ref> |
ANGPT2 is a competitive antagonist of TIE2 or a partial agonist of TIE2 depending on the context. In stressed ECs, one recent report suggests that ANGPT2 may activate TIE2 signaling in the absence of ANGPT1 and in high concentrations. | ANGPT2 is a competitive antagonist of TIE2 or a partial agonist of TIE2 depending on the context. In stressed ECs, one recent report suggests that ANGPT2 may activate TIE2 signaling in the absence of ANGPT1 and in high concentrations. | ||
| Line 23: | Line 23: | ||
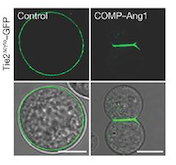
[[Image:controlANG.png]] | [[Image:controlANG.png]] | ||
| - | ''Fig 1. These cells express Tie2 and are marked with GFP. They were incubated with vehicle (control; left column) and COMP–Ang1 (right column). The scale bars represent 10 μm.''<ref name=" | + | ''Fig 1. These cells express Tie2 and are marked with GFP. They were incubated with vehicle (control; left column) and COMP–Ang1 (right column). The scale bars represent 10 μm.''<ref name="Angiopoietin 2"/> |
===• Signal transduction and kinase activity=== | ===• Signal transduction and kinase activity=== | ||
Revision as of 14:32, 11 January 2019
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
Crystal structure of cytoplasmic kinase domain of Tie2 in complex with decipera compound DP1919
| |||||||||||
References
- ↑ Fukuhara S, Sako K, Minami T, Noda K, Kim HZ, Kodama T, Shibuya M, Takakura N, Koh GY, Mochizuki N. Differential function of Tie2 at cell-cell contacts and cell-substratum contacts regulated by angiopoietin-1. Nat Cell Biol. 2008 May;10(5):513-26. doi: 10.1038/ncb1714. Epub 2008 Apr 20. PMID:18425120 doi:10.1038/ncb1714
- ↑ 2.0 2.1 Yuan HT, Khankin EV, Karumanchi SA, Parikh SM. Angiopoietin 2 is a partial agonist/antagonist of Tie2 signaling in the endothelium. Mol Cell Biol. 2009 Apr;29(8):2011-22. doi: 10.1128/MCB.01472-08. Epub 2009 Feb, 17. PMID:19223473 doi:10.1128/MCB.01472-08
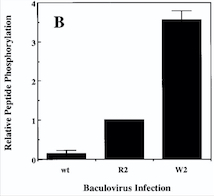
- ↑ 3.0 3.1 Murray BW, Padrique ES, Pinko C, McTigue MA. Mechanistic effects of autophosphorylation on receptor tyrosine kinase catalysis: enzymatic characterization of Tie2 and phospho-Tie2. Biochemistry. 2001 Aug 28;40(34):10243-53. PMID:11513602
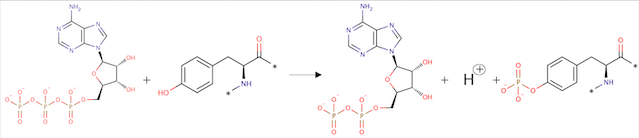
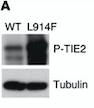
- ↑ Jones N, Chen SH, Sturk C, Master Z, Tran J, Kerbel RS, Dumont DJ. A unique autophosphorylation site on Tie2/Tek mediates Dok-R phosphotyrosine binding domain binding and function. Mol Cell Biol. 2003 Apr;23(8):2658-68. PMID:12665569
- ↑ Jones N, Chen SH, Sturk C, Master Z, Tran J, Kerbel RS, Dumont DJ. A unique autophosphorylation site on Tie2/Tek mediates Dok-R phosphotyrosine binding domain binding and function. Mol Cell Biol. 2003 Apr;23(8):2658-68. PMID:12665569
- ↑ Jones N, Chen SH, Sturk C, Master Z, Tran J, Kerbel RS, Dumont DJ. A unique autophosphorylation site on Tie2/Tek mediates Dok-R phosphotyrosine binding domain binding and function. Mol Cell Biol. 2003 Apr;23(8):2658-68. PMID:12665569
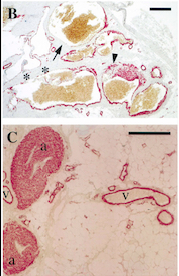
- ↑ Vikkula M, Boon LM, Carraway KL 3rd, Calvert JT, Diamonti AJ, Goumnerov B, Pasyk KA, Marchuk DA, Warman ML, Cantley LC, Mulliken JB, Olsen BR. Vascular dysmorphogenesis caused by an activating mutation in the receptor tyrosine kinase TIE2. Cell. 1996 Dec 27;87(7):1181-90. PMID:8980225
- ↑ Vikkula M, Boon LM, Carraway KL 3rd, Calvert JT, Diamonti AJ, Goumnerov B, Pasyk KA, Marchuk DA, Warman ML, Cantley LC, Mulliken JB, Olsen BR. Vascular dysmorphogenesis caused by an activating mutation in the receptor tyrosine kinase TIE2. Cell. 1996 Dec 27;87(7):1181-90. PMID:8980225
- ↑ Vikkula M, Boon LM, Carraway KL 3rd, Calvert JT, Diamonti AJ, Goumnerov B, Pasyk KA, Marchuk DA, Warman ML, Cantley LC, Mulliken JB, Olsen BR. Vascular dysmorphogenesis caused by an activating mutation in the receptor tyrosine kinase TIE2. Cell. 1996 Dec 27;87(7):1181-90. PMID:8980225
- ↑ Kangas J, Natynki M, Eklund L. Development of Molecular Therapies for Venous Malformations. Basic Clin Pharmacol Toxicol. 2018 Sep;123 Suppl 5:6-19. doi: 10.1111/bcpt.13027., Epub 2018 May 29. PMID:29668117 doi:http://dx.doi.org/10.1111/bcpt.13027
- ↑ Kangas J, Natynki M, Eklund L. Development of Molecular Therapies for Venous Malformations. Basic Clin Pharmacol Toxicol. 2018 Sep;123 Suppl 5:6-19. doi: 10.1111/bcpt.13027., Epub 2018 May 29. PMID:29668117 doi:http://dx.doi.org/10.1111/bcpt.13027
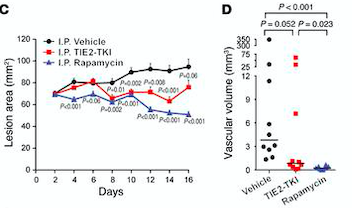
- ↑ Boscolo E, Limaye N, Huang L, Kang KT, Soblet J, Uebelhoer M, Mendola A, Natynki M, Seront E, Dupont S, Hammer J, Legrand C, Brugnara C, Eklund L, Vikkula M, Bischoff J, Boon LM. Rapamycin improves TIE2-mutated venous malformation in murine model and human subjects. J Clin Invest. 2015 Sep;125(9):3491-504. doi: 10.1172/JCI76004. Epub 2015 Aug 10. PMID:26258417 doi:http://dx.doi.org/10.1172/JCI76004
- ↑ Boscolo E, Limaye N, Huang L, Kang KT, Soblet J, Uebelhoer M, Mendola A, Natynki M, Seront E, Dupont S, Hammer J, Legrand C, Brugnara C, Eklund L, Vikkula M, Bischoff J, Boon LM. Rapamycin improves TIE2-mutated venous malformation in murine model and human subjects. J Clin Invest. 2015 Sep;125(9):3491-504. doi: 10.1172/JCI76004. Epub 2015 Aug 10. PMID:26258417 doi:http://dx.doi.org/10.1172/JCI76004
- ↑ Boscolo E, Limaye N, Huang L, Kang KT, Soblet J, Uebelhoer M, Mendola A, Natynki M, Seront E, Dupont S, Hammer J, Legrand C, Brugnara C, Eklund L, Vikkula M, Bischoff J, Boon LM. Rapamycin improves TIE2-mutated venous malformation in murine model and human subjects. J Clin Invest. 2015 Sep;125(9):3491-504. doi: 10.1172/JCI76004. Epub 2015 Aug 10. PMID:26258417 doi:http://dx.doi.org/10.1172/JCI76004
- ↑ Shlamkovich T, Aharon L, Koslawsky D, Einav Y, Papo N. Targeting the Tie2-alphavbeta3 integrin axis with bi-specific reagents for the inhibition of angiogenesis. BMC Biol. 2018 Aug 17;16(1):92. doi: 10.1186/s12915-018-0557-9. PMID:30119679 doi:http://dx.doi.org/10.1186/s12915-018-0557-9