We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1490
From Proteopedia
(Difference between revisions)
| Line 30: | Line 30: | ||
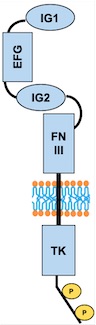
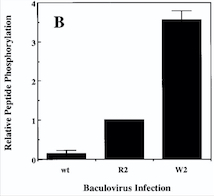
The activation of the receptor is due to a ligand-induced dimerization : the extracellular receptor domain dimerization brings the cytosolic kinase domains next to each other for intermolecular autophosphorylation. The latter occurs when one subunit of the dimeric receptor phosphorylates tyrosine residues on the other subunit. It happens in a sequential manner : Tyr-992 in the kinase activation loop is phosphorylated first, followed by autophosphorylation at Tyr-1108 and at additional tyrosine residues. Autophosphorylation also has multiple functions including recruitment of downstream signaling molecules.<ref name="Mechanistic effects of autophosphorylation" /> | The activation of the receptor is due to a ligand-induced dimerization : the extracellular receptor domain dimerization brings the cytosolic kinase domains next to each other for intermolecular autophosphorylation. The latter occurs when one subunit of the dimeric receptor phosphorylates tyrosine residues on the other subunit. It happens in a sequential manner : Tyr-992 in the kinase activation loop is phosphorylated first, followed by autophosphorylation at Tyr-1108 and at additional tyrosine residues. Autophosphorylation also has multiple functions including recruitment of downstream signaling molecules.<ref name="Mechanistic effects of autophosphorylation" /> | ||
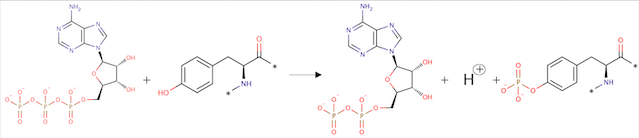
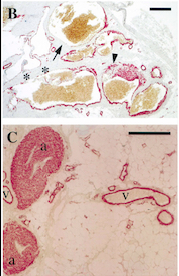
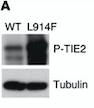
| - | Looking more closely at the TIE2 intracellular domain, 1106 is found at the base of a loop formed between the C-terminus tail and the C-terminus lobe of the kinase. The OH group of Tyr-1106 is thus directly into the solvent and accessible to phosphorylation. However, Tyr-1100 is not solvent exposed : thereby implying that the carboxy-terminal tail must undergo a conformational change upon activation of the receptor to expose this tyrosine residue for phosphorylation.<ref>PMID:12665569</ref> | + | Looking more closely at the TIE2 intracellular domain, 1106 is found at the base of a loop formed between the C-terminus tail and the C-terminus lobe of the kinase. The OH group of Tyr-1106 is thus directly into the solvent and accessible to phosphorylation. However, Tyr-1100 is not solvent exposed : thereby implying that the carboxy-terminal tail must undergo a conformational change upon activation of the receptor to expose this tyrosine residue for phosphorylation.<ref name="A unique autophosphorylation">PMID: 12665569</ref> |
| - | Consequent to ANGPT1 stimulation, the SH2 domain-containing p85 subunit of [[2pna|phosphatidylinositol (PI) 3-kinase]] is recruited to TIE via tyrosine residue 1100 in the C-end tail of the receptor, leading to activation of the enzyme.<ref | + | Consequent to ANGPT1 stimulation, the SH2 domain-containing p85 subunit of [[2pna|phosphatidylinositol (PI) 3-kinase]] is recruited to TIE via tyrosine residue 1100 in the C-end tail of the receptor, leading to activation of the enzyme.<ref name="A unique autophosphorylation"/> |
| - | Interestingly, inhibition of PI 3′ kinase activity can only partially inhibit the chemotactic effect of ANGPT1 on endothelial cells, thereby implying that additional TIE2 binding partners may also contribute to ANGPT1-mediated endothelial cell migration. Phosphorylation of TIE2 further results in its association with a docking protein related to downstream of kinase (Dok), known as Dok-R, it allows Dok-R to serve as a substrate of TIE2 and thereby become tyrosine phosphorylated.<ref | + | Interestingly, inhibition of PI 3′ kinase activity can only partially inhibit the chemotactic effect of ANGPT1 on endothelial cells, thereby implying that additional TIE2 binding partners may also contribute to ANGPT1-mediated endothelial cell migration. Phosphorylation of TIE2 further results in its association with a docking protein related to downstream of kinase (Dok), known as Dok-R, it allows Dok-R to serve as a substrate of TIE2 and thereby become tyrosine phosphorylated.<ref name="A unique autophosphorylation"/> |
Revision as of 14:34, 11 January 2019
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
Crystal structure of cytoplasmic kinase domain of Tie2 in complex with decipera compound DP1919
| |||||||||||
References
- ↑ Fukuhara S, Sako K, Minami T, Noda K, Kim HZ, Kodama T, Shibuya M, Takakura N, Koh GY, Mochizuki N. Differential function of Tie2 at cell-cell contacts and cell-substratum contacts regulated by angiopoietin-1. Nat Cell Biol. 2008 May;10(5):513-26. doi: 10.1038/ncb1714. Epub 2008 Apr 20. PMID:18425120 doi:10.1038/ncb1714
- ↑ 2.0 2.1 Yuan HT, Khankin EV, Karumanchi SA, Parikh SM. Angiopoietin 2 is a partial agonist/antagonist of Tie2 signaling in the endothelium. Mol Cell Biol. 2009 Apr;29(8):2011-22. doi: 10.1128/MCB.01472-08. Epub 2009 Feb, 17. PMID:19223473 doi:10.1128/MCB.01472-08
- ↑ 3.0 3.1 Murray BW, Padrique ES, Pinko C, McTigue MA. Mechanistic effects of autophosphorylation on receptor tyrosine kinase catalysis: enzymatic characterization of Tie2 and phospho-Tie2. Biochemistry. 2001 Aug 28;40(34):10243-53. PMID:11513602
- ↑ 4.0 4.1 4.2 Jones N, Chen SH, Sturk C, Master Z, Tran J, Kerbel RS, Dumont DJ. A unique autophosphorylation site on Tie2/Tek mediates Dok-R phosphotyrosine binding domain binding and function. Mol Cell Biol. 2003 Apr;23(8):2658-68. PMID:12665569
- ↑ Vikkula M, Boon LM, Carraway KL 3rd, Calvert JT, Diamonti AJ, Goumnerov B, Pasyk KA, Marchuk DA, Warman ML, Cantley LC, Mulliken JB, Olsen BR. Vascular dysmorphogenesis caused by an activating mutation in the receptor tyrosine kinase TIE2. Cell. 1996 Dec 27;87(7):1181-90. PMID:8980225
- ↑ Vikkula M, Boon LM, Carraway KL 3rd, Calvert JT, Diamonti AJ, Goumnerov B, Pasyk KA, Marchuk DA, Warman ML, Cantley LC, Mulliken JB, Olsen BR. Vascular dysmorphogenesis caused by an activating mutation in the receptor tyrosine kinase TIE2. Cell. 1996 Dec 27;87(7):1181-90. PMID:8980225
- ↑ Vikkula M, Boon LM, Carraway KL 3rd, Calvert JT, Diamonti AJ, Goumnerov B, Pasyk KA, Marchuk DA, Warman ML, Cantley LC, Mulliken JB, Olsen BR. Vascular dysmorphogenesis caused by an activating mutation in the receptor tyrosine kinase TIE2. Cell. 1996 Dec 27;87(7):1181-90. PMID:8980225
- ↑ Kangas J, Natynki M, Eklund L. Development of Molecular Therapies for Venous Malformations. Basic Clin Pharmacol Toxicol. 2018 Sep;123 Suppl 5:6-19. doi: 10.1111/bcpt.13027., Epub 2018 May 29. PMID:29668117 doi:http://dx.doi.org/10.1111/bcpt.13027
- ↑ Kangas J, Natynki M, Eklund L. Development of Molecular Therapies for Venous Malformations. Basic Clin Pharmacol Toxicol. 2018 Sep;123 Suppl 5:6-19. doi: 10.1111/bcpt.13027., Epub 2018 May 29. PMID:29668117 doi:http://dx.doi.org/10.1111/bcpt.13027
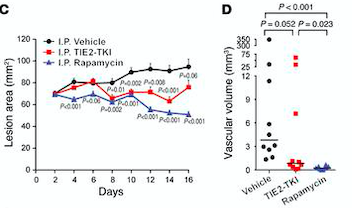
- ↑ Boscolo E, Limaye N, Huang L, Kang KT, Soblet J, Uebelhoer M, Mendola A, Natynki M, Seront E, Dupont S, Hammer J, Legrand C, Brugnara C, Eklund L, Vikkula M, Bischoff J, Boon LM. Rapamycin improves TIE2-mutated venous malformation in murine model and human subjects. J Clin Invest. 2015 Sep;125(9):3491-504. doi: 10.1172/JCI76004. Epub 2015 Aug 10. PMID:26258417 doi:http://dx.doi.org/10.1172/JCI76004
- ↑ Boscolo E, Limaye N, Huang L, Kang KT, Soblet J, Uebelhoer M, Mendola A, Natynki M, Seront E, Dupont S, Hammer J, Legrand C, Brugnara C, Eklund L, Vikkula M, Bischoff J, Boon LM. Rapamycin improves TIE2-mutated venous malformation in murine model and human subjects. J Clin Invest. 2015 Sep;125(9):3491-504. doi: 10.1172/JCI76004. Epub 2015 Aug 10. PMID:26258417 doi:http://dx.doi.org/10.1172/JCI76004
- ↑ Boscolo E, Limaye N, Huang L, Kang KT, Soblet J, Uebelhoer M, Mendola A, Natynki M, Seront E, Dupont S, Hammer J, Legrand C, Brugnara C, Eklund L, Vikkula M, Bischoff J, Boon LM. Rapamycin improves TIE2-mutated venous malformation in murine model and human subjects. J Clin Invest. 2015 Sep;125(9):3491-504. doi: 10.1172/JCI76004. Epub 2015 Aug 10. PMID:26258417 doi:http://dx.doi.org/10.1172/JCI76004
- ↑ Shlamkovich T, Aharon L, Koslawsky D, Einav Y, Papo N. Targeting the Tie2-alphavbeta3 integrin axis with bi-specific reagents for the inhibition of angiogenesis. BMC Biol. 2018 Aug 17;16(1):92. doi: 10.1186/s12915-018-0557-9. PMID:30119679 doi:http://dx.doi.org/10.1186/s12915-018-0557-9