We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1490
From Proteopedia
(Difference between revisions)
| Line 220: | Line 220: | ||
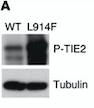
– '''Mutation in position 849 : Arginine → Tryptophane:''' Change from large size and basic (R) to large size and aromatic (W). Increased autophosphorylation and kinase activation; no effect on location at membrane. | – '''Mutation in position 849 : Arginine → Tryptophane:''' Change from large size and basic (R) to large size and aromatic (W). Increased autophosphorylation and kinase activation; no effect on location at membrane. | ||
| - | Arginine at position 849 is found in six residues upstream of the invariant lysine K855 in the kinase domain (sequence preserved among the human, bovine, murine and rat TIE2 sequences). This seems to prove that a basic amino acid is essential for this position. In addition, arginine located a few amino acids before invariant lysine is involved in stabilizing the kinase domain (hydrogen binding of arginine with a proline downstream). It is therefore possible that R849 may also be involved in the stabilization of the kinase domain. Thus, the substitution of R849 by a W could modify the conformation of the kinase domain, leading to a decrease in inhibitory mechanisms and involving autophosphorylation.<ref>PMID:8980225</ref> | + | Arginine at position 849 is found in six residues upstream of the invariant lysine K855 in the kinase domain (sequence preserved among the human, bovine, murine and rat TIE2 sequences). This seems to prove that a basic amino acid is essential for this position. In addition, arginine located a few amino acids before invariant lysine is involved in stabilizing the kinase domain (hydrogen binding of arginine with a proline downstream). It is therefore possible that R849 may also be involved in the stabilization of the kinase domain. Thus, the substitution of R849 by a W could modify the conformation of the kinase domain, leading to a decrease in inhibitory mechanisms and involving autophosphorylation.<ref name="Vascular dysmorphogenesis">PMID: 8980225</ref> |
[[Image:Venous Malformations Diagram.jpg]] | [[Image:Venous Malformations Diagram.jpg]] | ||
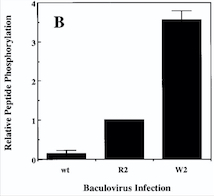
| - | ''Fig 4. Diagram : Comparison of the Kinase Activities of Normal and Mutant TIE2 Receptors. (B) Cells infected with wild-type baculovirus (wt) or virus expressing normal TIE2 (R2) or mutant TIE2 (W2). Cells expressing the mutation at position 849 (Arginine → Tryptophan) have an autophosphorylation activity 6 to 10 times higher than wild cells.''<ref | + | ''Fig 4. Diagram : Comparison of the Kinase Activities of Normal and Mutant TIE2 Receptors. (B) Cells infected with wild-type baculovirus (wt) or virus expressing normal TIE2 (R2) or mutant TIE2 (W2). Cells expressing the mutation at position 849 (Arginine → Tryptophan) have an autophosphorylation activity 6 to 10 times higher than wild cells.''<ref name="Vascular dysmorphogenesis"/> |
| Line 231: | Line 231: | ||
[[Image:Venous Malformations Immunohistochemistry.jpg]] | [[Image:Venous Malformations Immunohistochemistry.jpg]] | ||
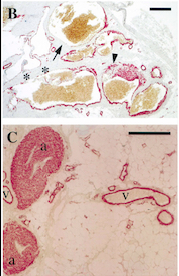
| - | ''Fig 5. Pictures of immunohistochemistry of VMs with Antibodies against Smooth Muscle Cells 𝛂-Actin <ref | + | ''Fig 5. Pictures of immunohistochemistry of VMs with Antibodies against Smooth Muscle Cells 𝛂-Actin <ref name="Vascular dysmorphogenesis"/>'' |
''B = Abnormal channels'' | ''B = Abnormal channels'' | ||
Revision as of 14:37, 11 January 2019
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
Crystal structure of cytoplasmic kinase domain of Tie2 in complex with decipera compound DP1919
| |||||||||||
References
- ↑ Fukuhara S, Sako K, Minami T, Noda K, Kim HZ, Kodama T, Shibuya M, Takakura N, Koh GY, Mochizuki N. Differential function of Tie2 at cell-cell contacts and cell-substratum contacts regulated by angiopoietin-1. Nat Cell Biol. 2008 May;10(5):513-26. doi: 10.1038/ncb1714. Epub 2008 Apr 20. PMID:18425120 doi:10.1038/ncb1714
- ↑ 2.0 2.1 Yuan HT, Khankin EV, Karumanchi SA, Parikh SM. Angiopoietin 2 is a partial agonist/antagonist of Tie2 signaling in the endothelium. Mol Cell Biol. 2009 Apr;29(8):2011-22. doi: 10.1128/MCB.01472-08. Epub 2009 Feb, 17. PMID:19223473 doi:10.1128/MCB.01472-08
- ↑ 3.0 3.1 Murray BW, Padrique ES, Pinko C, McTigue MA. Mechanistic effects of autophosphorylation on receptor tyrosine kinase catalysis: enzymatic characterization of Tie2 and phospho-Tie2. Biochemistry. 2001 Aug 28;40(34):10243-53. PMID:11513602
- ↑ 4.0 4.1 4.2 Jones N, Chen SH, Sturk C, Master Z, Tran J, Kerbel RS, Dumont DJ. A unique autophosphorylation site on Tie2/Tek mediates Dok-R phosphotyrosine binding domain binding and function. Mol Cell Biol. 2003 Apr;23(8):2658-68. PMID:12665569
- ↑ 5.0 5.1 5.2 Vikkula M, Boon LM, Carraway KL 3rd, Calvert JT, Diamonti AJ, Goumnerov B, Pasyk KA, Marchuk DA, Warman ML, Cantley LC, Mulliken JB, Olsen BR. Vascular dysmorphogenesis caused by an activating mutation in the receptor tyrosine kinase TIE2. Cell. 1996 Dec 27;87(7):1181-90. PMID:8980225
- ↑ Kangas J, Natynki M, Eklund L. Development of Molecular Therapies for Venous Malformations. Basic Clin Pharmacol Toxicol. 2018 Sep;123 Suppl 5:6-19. doi: 10.1111/bcpt.13027., Epub 2018 May 29. PMID:29668117 doi:http://dx.doi.org/10.1111/bcpt.13027
- ↑ Kangas J, Natynki M, Eklund L. Development of Molecular Therapies for Venous Malformations. Basic Clin Pharmacol Toxicol. 2018 Sep;123 Suppl 5:6-19. doi: 10.1111/bcpt.13027., Epub 2018 May 29. PMID:29668117 doi:http://dx.doi.org/10.1111/bcpt.13027
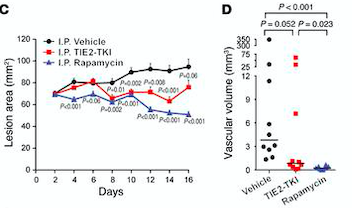
- ↑ Boscolo E, Limaye N, Huang L, Kang KT, Soblet J, Uebelhoer M, Mendola A, Natynki M, Seront E, Dupont S, Hammer J, Legrand C, Brugnara C, Eklund L, Vikkula M, Bischoff J, Boon LM. Rapamycin improves TIE2-mutated venous malformation in murine model and human subjects. J Clin Invest. 2015 Sep;125(9):3491-504. doi: 10.1172/JCI76004. Epub 2015 Aug 10. PMID:26258417 doi:http://dx.doi.org/10.1172/JCI76004
- ↑ Boscolo E, Limaye N, Huang L, Kang KT, Soblet J, Uebelhoer M, Mendola A, Natynki M, Seront E, Dupont S, Hammer J, Legrand C, Brugnara C, Eklund L, Vikkula M, Bischoff J, Boon LM. Rapamycin improves TIE2-mutated venous malformation in murine model and human subjects. J Clin Invest. 2015 Sep;125(9):3491-504. doi: 10.1172/JCI76004. Epub 2015 Aug 10. PMID:26258417 doi:http://dx.doi.org/10.1172/JCI76004
- ↑ Boscolo E, Limaye N, Huang L, Kang KT, Soblet J, Uebelhoer M, Mendola A, Natynki M, Seront E, Dupont S, Hammer J, Legrand C, Brugnara C, Eklund L, Vikkula M, Bischoff J, Boon LM. Rapamycin improves TIE2-mutated venous malformation in murine model and human subjects. J Clin Invest. 2015 Sep;125(9):3491-504. doi: 10.1172/JCI76004. Epub 2015 Aug 10. PMID:26258417 doi:http://dx.doi.org/10.1172/JCI76004
- ↑ Shlamkovich T, Aharon L, Koslawsky D, Einav Y, Papo N. Targeting the Tie2-alphavbeta3 integrin axis with bi-specific reagents for the inhibition of angiogenesis. BMC Biol. 2018 Aug 17;16(1):92. doi: 10.1186/s12915-018-0557-9. PMID:30119679 doi:http://dx.doi.org/10.1186/s12915-018-0557-9