Sandbox Reserved 1500
From Proteopedia
| Line 1: | Line 1: | ||
{{Sandbox_Reserved_ESBS}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | {{Sandbox_Reserved_ESBS}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
| + | <Structure load='5hfg' size='450' frame='true' align='right' caption='' scene='Insert optional scene name here' /> | ||
== Structural highlights == | == Structural highlights == | ||
| Line 23: | Line 24: | ||
| - | + | ||
== Nature == | == Nature == | ||
Revision as of 15:46, 11 January 2019
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
|
Contents |
Structural highlights
| 5hfg is a 1 chain structure. Full crystallographic information is available from OCA. For a guided tour on the structure components use FirstGlance. | |
| Related: | 5hfi |
| Secondary structure: | 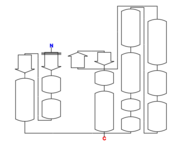 Figure 1: 5HFG secondary structure.[1] |
| Resources: | FirstGlance, OCA, PDBe, RCSB, PDBsum, ProSAT |
Global Symmetry: Asymmetric - C1
Global Stoichiometry: Monomer - A
Its theoretical weight is 25.29 KDa
Primary structure
5hfg is a 1 chain structure of 238 amino acids. [2]
Secondary structure
The structure of 5hfg mainly consists in alpha helix (12) , beta sheets (4), you can check the 3D view here. 
Tertiary structure
Monomeric assembly composition. 5hfg is one distinct polypeptide molecule. [3]
Nature
5hfg is a Dsb-family protein, from Pseudomonas Aeruginosa. This family of proteins is mainly used to oxidize and reduce cysteine residues of substrate proteins. Most enzymes from Dsb-family catalyze disulfide formation in periplasmic or secreted substrate proteins. [4] It has no bound ligands and no modified residues. Sequence domains: Thioredoxin-like superfamily DSBA-like thioredoxin domain
Function
This protein has a disulfide oxidoreductase activity.
Relevance
Click to see a sample scene created with SAT by Group, and to see a transparent representation of the protein.
