Sandbox Reserved 1496
From Proteopedia
Revision as of 16:57, 11 January 2019
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
|
Contents |
Presentation of dehydrosqualene synthase
The C30 carotene synthase (also known as 4,4'-diapophytoene (DAP) synthase; dehydrosqualene synthase; CrtM), encoded by crtM gene, is a bacterial carotenoid synthase which is involved in the first step of the pathway that synthesizes staphyloxanthin from farnesyl diphosphate.
This pathway is a subpathway in staphyloxanthin biosynthesis, which is itself part of carotenoid biosynthesis. Carotenoid pathways are branches of the general isoprenoid pathway.
Staphyloxanthin is a carotenoid, which is responsible for the signature golden color of Staphylococcus aureus and also plays the role of virulence factor to human. It has an antioxidant activity that helps the microbe to survive under hight concentration of reactive oxygen species, caused by the host immune system. Having a better comprehension of the synthesis of this carotenoid will help to find a cure to S. aureus related diseases.
Function(Biological reaction)
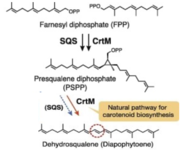
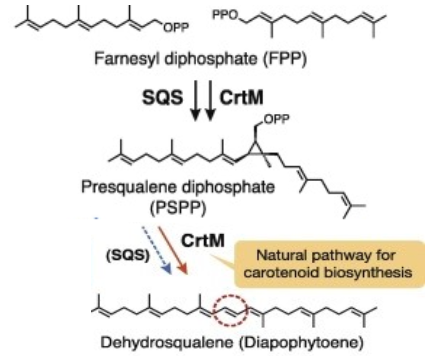
CrtM catalyses the first step of the synthesis of staphyloxanthin (with a 2-step mechanism)[2][1].
- Catalysed reaction (head-to-head condensation)
- 2 (2E,6E)-farnesyl diphosphate (FPP) ↔15-cis-4,4'-diapophytoene + 2 diphosphate
The gene is located in the staphyloxanthin biosynthesis operon (crtOPQMN), which encodes enzymes to catalyze the synthesis[3]. The product, C(30) carotenoid 4,4'-diapophytoene (dehydrosqualene), is colorless.
Transferase Activity : Transferring alkyl or aryl groups, other than methyl groups
Metal Ion Binding : requires Mn2+ as cofactor (2 ions per subunit).
Structure
This enzyme consists of 2 sequence-identical polypeptide chains of 287 amino acids[4] and is biologically active as a dimerTemplate:Cn.
- Ligands
- 6 Magnesium ions
- 2 PO42-
- 1 L(+)-tartaric acid
- 2 (3r)-3-biphenyl-4-yl-1-azabicyclo[2.2.2]octan-3-ol
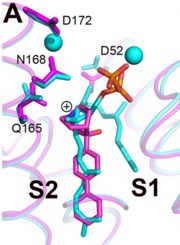
Structures of BPH-651, a 3-OH quinuclidine inhibitor, bound to CrtM
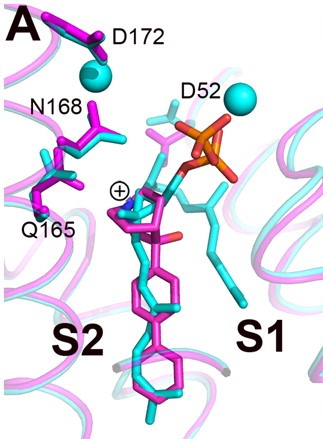 [5]. Three phosphonosulfonates, which mimic the FPP substract have been identified to have binding/inhibiting activity on CrtM, each with different binding pattern.
[5]. Three phosphonosulfonates, which mimic the FPP substract have been identified to have binding/inhibiting activity on CrtM, each with different binding pattern.
Human diseases
The Staphyloxanthin catalyzed by CrtM is a virulence factor linked with infections caused by methicillin-resistant Staphylococcus aureus (MRSA), which is becoming a serious threat[6]. Cells with mutated CrtM are less resistant against oxidative stress, shows lower survival rate and are less pathogenic in mouses[7]. Due to its structural similarity as well as shared inhibitor with human squalene synthase (SQS)[8][9], it is seen as a potential target for virulence factor neutralization therapy, where the idea is to inhibit the pathogen's synthetic pathway. Drugs intended to lower the cholesterol level might have a positive effect on fighting the infection[1]. Cationic inhibitors are here of interest as anti-infectives because they can substitute themselves to Mg2+ and inactivate the enzyme.
Links
References
- ↑ 1.0 1.1 1.2 Song Y, Liu CI, Lin FY, No JH, Hensler M, Liu YL, Jeng WY, Low J, Liu GY, Nizet V, Wang AH, Oldfield E. Inhibition of staphyloxanthin virulence factor biosynthesis in Staphylococcus aureus: in vitro, in vivo, and crystallographic results. J Med Chem. 2009 Jul 9;52(13):3869-80. PMID:19456099 doi:10.1021/jm9001764
- ↑ Clauditz A, Resch A, Wieland KP, Peschel A, Gotz F. Staphyloxanthin plays a role in the fitness of Staphylococcus aureus and its ability to cope with oxidative stress. Infect Immun. 2006 Aug;74(8):4950-3. doi: 10.1128/IAI.00204-06. PMID:16861688 doi:http://dx.doi.org/10.1128/IAI.00204-06
- ↑ Pelz A, Wieland KP, Putzbach K, Hentschel P, Albert K, Gotz F. Structure and biosynthesis of staphyloxanthin from Staphylococcus aureus. J Biol Chem. 2005 Sep 16;280(37):32493-8. doi: 10.1074/jbc.M505070200. Epub 2005 , Jul 14. PMID:16020541 doi:http://dx.doi.org/10.1074/jbc.M505070200
- ↑ Aswani V, Mau B, Shukla SK. Complete Genome Sequence of Staphylococcus aureus MCRF184, a Necrotizing Fasciitis-Causing Methicillin-Sensitive Sequence Type 45 Staphylococcus Strain. Genome Announc. 2016 May 12;4(3). pii: 4/3/e00374-16. doi:, 10.1128/genomeA.00374-16. PMID:27174283 doi:http://dx.doi.org/10.1128/genomeA.00374-16
- ↑ Mechanism of action and inhibition of dehydrosqualene synthase Fu-Yang Lin, Chia-I Liu, Yi-Liang Liu, Yonghui Zhang, Ke Wang, Wen-Yih Jeng, Tzu-Ping Ko, Rong Cao, Andrew H.-J. Wang, and Eric Oldfield PNAS December 14, 2010 107 (50) 21337-21342; https://doi.org/10.1073/pnas.1010907107<ref></ref> The space group of FsPP·CrtM is P3121, with two molecules per asymmetric unit<ref>PMID: 18276850</li> <li id="cite_note-5">[[#cite_ref-5|↑]] Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007 Oct 17;298(15):1763-71. doi: 10.1001/jama.298.15.1763. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/17940231 17940231] doi:[http://dx.doi.org/10.1001/jama.298.15.1763 http://dx.doi.org/10.1001/jama.298.15.1763]</li> <li id="cite_note-6">[[#cite_ref-6|↑]] Liu GY, Essex A, Buchanan JT, Datta V, Hoffman HM, Bastian JF, Fierer J, Nizet V. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J Exp Med. 2005 Jul 18;202(2):209-15. doi: 10.1084/jem.20050846. Epub 2005 Jul, 11. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/16009720 16009720] doi:[http://dx.doi.org/10.1084/jem.20050846 http://dx.doi.org/10.1084/jem.20050846]</li> <li id="cite_note-ACBI">[[#cite_ref-ACBI_0|↑]] <strong class="error">Cite error: Invalid <code><ref></code> tag; no text was provided for refs named <code>ACBI</code></strong></li> <li id="cite_note-8">[[#cite_ref-8|↑]] Lin FY, Liu YL, Li K, Cao R, Zhu W, Axelson J, Pang R, Oldfield E. Head-to-Head Prenyl Tranferases: Anti-Infective Drug Targets. J Med Chem. 2012 May 10;55(9):4367-72. Epub 2012 May 1. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/22486710 22486710] doi:[http://dx.doi.org/10.1021/jm300208p 10.1021/jm300208p]</li></ol></ref>