Sandbox Reserved 1493
From Proteopedia
| Line 2: | Line 2: | ||
== Integrin αIIbβ3 Headpiece (2VDL) == | == Integrin αIIbβ3 Headpiece (2VDL) == | ||
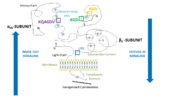
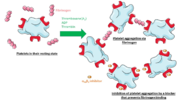
| - | '''Integrin αIIbβ3''' (or glycoprotein IIb/IIIa) is a complex present on the membrane of platelets that intervenes in the activation, adherence and aggregation of platelets during clotting. It is a cation-dependant heterodimeric transmembrane receptor containing a large extracellular headpiece and short intracellular tails. It is synthesized in megakaryocytes. | + | '''Integrin αIIbβ3''' (or glycoprotein IIb/IIIa) is a complex present on the membrane of platelets that intervenes in the activation, adherence and aggregation of platelets during '''clotting'''. It is a cation-dependant heterodimeric transmembrane receptor containing a large extracellular headpiece and short intracellular tails. It is synthesized in megakaryocytes. |
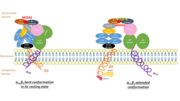
| - | Its particular shape and localisation on the membrane allows both transduction of the intracellular activation signal and extracellular ligand binding. It is the dominant integrin on platelets with 70,000 to 90,000 receptors expressed on each platelet in the resting state. | + | Its particular shape and localisation on the membrane allows both transduction of the intracellular activation signal and extracellular ligand binding. It is the dominant integrin on '''platelets''' with 70,000 to 90,000 receptors expressed on each platelet in the resting state. |
The headpiece (2VDL) of integrin αIIbβ3 enables cation-facilitated ligand binding with multiple ligands (most known being [[fibrinogen]], [[fibronectin]], von Willebrand factors, [[thrombospondin]] and vitronectin). Binding affinity is dynamic and depends on the conformational status of the receptor. | The headpiece (2VDL) of integrin αIIbβ3 enables cation-facilitated ligand binding with multiple ligands (most known being [[fibrinogen]], [[fibronectin]], von Willebrand factors, [[thrombospondin]] and vitronectin). Binding affinity is dynamic and depends on the conformational status of the receptor. | ||
| Line 12: | Line 12: | ||
== Structure == | == Structure == | ||
| - | αIIbβ3 belongs to the integrin family: it is a bidirectional information mediator between the interior of the cell and its external environment. It has the ability to receive intracellular signals via the cytoplasmic tail and to bind ligands at the extracellular headpiece. | + | αIIbβ3 belongs to the '''integrin''' family: it is a bidirectional information mediator between the interior of the cell and its external environment. It has the ability to receive intracellular signals via the cytoplasmic tail and to bind ligands at the extracellular headpiece. |
| - | It is composed of adjacent αIIb and β3 subunits that are not covalently linked. Both have a small intracellular cytoplasmic tail domain and a single transmembrane domain which links the first one to a large N-terminal extracellular domain. The latter far exceeds the cytoplasmic domain in size | + | It is composed of adjacent '''αIIb and β3 subunits''' that are not covalently linked. Both have a small intracellular cytoplasmic tail domain and a single transmembrane domain which links the first one to a large N-terminal extracellular domain. The latter far exceeds the cytoplasmic domain in size an can bind high molecular weight ligands (fibrinogen is approximately 340 kDa). |
| - | Ligands bind in a groove between the integrin subunits, at the intersection of the integrin extracellular β-propeller of the αIIb subunit and the β I domain of the β3 subunit. There are multiple binding sites in both subunits. | + | Ligands bind in a groove between the integrin subunits, at the intersection of the integrin extracellular '''β-propeller of the αIIb subunit''' and the '''β I domain of the β3 subunit'''. There are multiple binding sites in both subunits. |
=== αIIb subunit === | === αIIb subunit === | ||
| - | The αIIb subunit (glycoprotein IIb) is composed of 1008 amino acids unevenly distributed over 2 chains. A part forms a light chain which comports a cytoplasmic tail of 20 amino acids and a transmembrane helix. An extracellular disulfide segment links this chain to the <scene name='80/802667/Alpha_head/1'>heavy extracellular domain</scene> which is part of the headpiece of the integrin. | + | The '''αIIb subunit''' (glycoprotein IIb) is composed of 1008 amino acids unevenly distributed over 2 chains. A part forms a light chain which comports a cytoplasmic tail of 20 amino acids and a transmembrane helix. An extracellular disulfide segment links this chain to the <scene name='80/802667/Alpha_head/1'>heavy extracellular domain</scene> which is part of the headpiece of the integrin. |
| - | The extracellular N-Terminus forms a cap over the β-propeller domain which is folded by seven successive blades of aminoterminal repeats. Each blade is a β-hairpin loop-like structure composed of 4 antiparallel β strands located in each repeat and connected by loops of the surface. This β-propeller is linked to a thigh and two calf domains, which form the leg structure that supports the heavy head. The total forms the stalk of the αIIb subunit. The | + | The extracellular N-Terminus forms a cap over the '''β-propeller''' domain which is folded by seven successive blades of aminoterminal repeats. Each blade is a β-hairpin loop-like structure composed of 4 antiparallel β strands located in each repeat and connected by loops of the surface. This β-propeller is linked to a '''thigh''' and '''two calf''' domains, which form the leg structure that supports the heavy head. The total forms the stalk of the αIIb subunit. The knee of the subunit between the thigh and the first calf domain is the site at which the head bends (inactivated form of the integrin. |
| - | The β-propeller hosts multiple cation biding sites. The last 3 or 4 blades bind <scene name='80/802667/Ca_ions_on_beta-propeller/3'>Ca2+ ions</scene> which influence ligand binding on the lower side of the blades and | + | The β-propeller hosts multiple '''cation biding sites'''. The last 3 or 4 blades bind <scene name='80/802667/Ca_ions_on_beta-propeller/3'>'''Ca2+ ions'''</scene> which influence ligand binding on the lower side of the blades and play an important role in biogenesis and stability of the heterodimer. The '''I domain''' inserted between blades 2 and 3 in the β-propeller follows a Rossman fold with five β-sheets surrounded by seven α-helices. Ligand binding occurs between the β-propeller and the β I domain of the β3 subunit via a coordinating '''Mg2+ ion''' in the MIDAS of the β3 subunit. |
| - | The RGD binding site (Arg-Gly-Asp) is in a crevice in this region, inserted between the β-propeller and β I domains | + | The '''RGD binding site''' (Arg-Gly-Asp) is in a crevice in this region, inserted between the β-propeller and β I domains. |
=== β3 subunit === | === β3 subunit === | ||
| Line 32: | Line 32: | ||
The β3 subunit (glycoprotein IIIa) is a 762 amino acids polypeptide chain. | The β3 subunit (glycoprotein IIIa) is a 762 amino acids polypeptide chain. | ||
| - | Its <scene name='80/802667/Beta_head/1'>head</scene> is composed of a β I domain which has a fold similar to the I | + | Its <scene name='80/802667/Beta_head/1'>'''head'''</scene> is composed of a '''β I domain''' which has a fold similar to the I domain of the head of the α subunit. It has a <scene name='80/802667/Mg_in_beta_head_midas/1'>Mg2+</scene> coordinating '''metal ion dependent adhesion site (MIDAS)''' motif and a site adjacent to MIDAS ('''ADMIDAS''') which coordinates ions and plays a part in activity modulation. |
| - | Its stalk is mainly composed of a plexin-sempahorin-integrin (PSI) domain and | + | Its '''stalk''' is mainly composed of a plexin-sempahorin-integrin (PSI) domain and a '''hybrid domain'''. A '''cysteine-rich core''' occupies the extracellular part of β3 from residues 400 to 650. It is linked to the N-terminal of the protein thanks to a long-range disulfide bond. As the globular head is part of the ligand-binding headpiece (1 cation binding site, RGD and KGD binding sites), the cysteine-rich region is thought to have a role in the activation of the headpiece. |
| - | A cysteine-rich core occupies the extracellular part of β3 from residues 400 to 650. It is linked to the N-terminal of the protein thanks to a long-range disulfide bond. As the globular head is part of the ligand-binding headpiece (1 cation binding site, RGD and KGD binding sites), the cysteine-rich region is thought to have a role in the activation of the headpiece. | + | |
The cytoplasmic tail of the β3 subunit has a NPLY domain which bind proteins with phosphotyrosine binding (PTB) domains. | The cytoplasmic tail of the β3 subunit has a NPLY domain which bind proteins with phosphotyrosine binding (PTB) domains. | ||
| Line 122: | Line 121: | ||
Glanzmann thrombasthenia is a hemorrhagic disease characterized by a lack of αIIbβ3 complexes on the membrane of platelets, an absence of fibrinogen and an inability to retract a clot. This deficiency is caused by mutation in the sequence of the integrin. Multiple amino acids can be affected. Here are two examples of consequences: | Glanzmann thrombasthenia is a hemorrhagic disease characterized by a lack of αIIbβ3 complexes on the membrane of platelets, an absence of fibrinogen and an inability to retract a clot. This deficiency is caused by mutation in the sequence of the integrin. Multiple amino acids can be affected. Here are two examples of consequences: | ||
| - | * Pro-145 mutation at an Ala of the W3:4-1 loop and Leu-183 mutation at a Pro of a surrounding loop cause insertion of two amino acid residues into the first loop | + | * Pro-145 mutation at an Ala of the W3:4-1 loop(residues 147 to 166) and Leu-183 mutation at a Pro of a surrounding loop cause insertion of two amino acid residues into the first loop and modifies loop conformations. It results in a reduction in the expression level of integrin αIIbβ3. |
* mutation of the Arg 724 terminus of the β3 subunit which generates a truncated integrin composed only of the first 8 of the 47 amino acids normally present in its cytoplasmic domain. | * mutation of the Arg 724 terminus of the β3 subunit which generates a truncated integrin composed only of the first 8 of the 47 amino acids normally present in its cytoplasmic domain. | ||
Revision as of 18:15, 11 January 2019
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
Integrin αIIbβ3 Headpiece (2VDL)
Integrin αIIbβ3 (or glycoprotein IIb/IIIa) is a complex present on the membrane of platelets that intervenes in the activation, adherence and aggregation of platelets during clotting. It is a cation-dependant heterodimeric transmembrane receptor containing a large extracellular headpiece and short intracellular tails. It is synthesized in megakaryocytes.
Its particular shape and localisation on the membrane allows both transduction of the intracellular activation signal and extracellular ligand binding. It is the dominant integrin on platelets with 70,000 to 90,000 receptors expressed on each platelet in the resting state.
The headpiece (2VDL) of integrin αIIbβ3 enables cation-facilitated ligand binding with multiple ligands (most known being fibrinogen, fibronectin, von Willebrand factors, thrombospondin and vitronectin). Binding affinity is dynamic and depends on the conformational status of the receptor.
| |||||||||||
References
Structure and function:
- Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010 Jan;339(1):269-80. Epub 2009 Aug 20. PMID:19693543 doi:10.1007/s00441-009-0834-6
- Lefkovits J, Plow EF, Topol EJ. Platelet glycoprotein IIb/IIIa receptors in cardiovascular medicine. N Engl J Med. 1995 Jun 8;332(23):1553-9. doi: 10.1056/NEJM199506083322306. PMID:7739710 doi:http://dx.doi.org/10.1056/NEJM199506083322306
- Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010 Jan;339(1):269-80. Epub 2009 Aug 20. PMID:19693543 doi:10.1007/s00441-009-0834-6
- Podolnikova NP, Yakovlev S, Yakubenko VP, Wang X, Gorkun OV, Ugarova TP. The interaction of integrin alphaIIbbeta3 with fibrin occurs through multiple binding sites in the alphaIIb beta-propeller domain. J Biol Chem. 2014 Jan 24;289(4):2371-83. doi: 10.1074/jbc.M113.518126. Epub 2013 , Dec 12. PMID:24338009 doi:http://dx.doi.org/10.1074/jbc.M113.518126
- Chew DP, Moliterno DJ. A critical appraisal of platelet glycoprotein IIb/IIIa inhibition. J Am Coll Cardiol. 2000 Dec;36(7):2028-35. doi: 10.1016/s0735-1097(00)00979-7. PMID:11127436 doi:http://dx.doi.org/10.1016/s0735-1097(00)00979-7
- Kamata T, Tieu KK, Irie A, Springer TA, Takada Y. Amino acid residues in the alpha IIb subunit that are critical for ligand binding to integrin alpha IIbbeta 3 are clustered in the beta-propeller model. J Biol Chem. 2001 Nov 23;276(47):44275-83. Epub 2001 Sep 13. PMID:11557768 doi:10.1074/jbc.M107021200
Activity modulation:
- Hantgan RR, Stahle MC, Lord ST. Dynamic regulation of fibrinogen: integrin alphaIIbbeta3 binding. Biochemistry. 2010 Nov 2;49(43):9217-25. doi: 10.1021/bi1009858. PMID:20828133 doi:http://dx.doi.org/10.1021/bi1009858
- Podolnikova NP, Yakovlev S, Yakubenko VP, Wang X, Gorkun OV, Ugarova TP. The interaction of integrin alphaIIbbeta3 with fibrin occurs through multiple binding sites in the alphaIIb beta-propeller domain. J Biol Chem. 2014 Jan 24;289(4):2371-83. doi: 10.1074/jbc.M113.518126. Epub 2013 , Dec 12. PMID:24338009 doi:http://dx.doi.org/10.1074/jbc.M113.518126
- doi: https://dx.doi.org/https
- Joo SJ. Mechanisms of Platelet Activation and Integrin alphaIIbeta3. Korean Circ J. 2012 May;42(5):295-301. doi: 10.4070/kcj.2012.42.5.295. Epub 2012 , May 24. PMID:22701130 doi:http://dx.doi.org/10.4070/kcj.2012.42.5.295
- Yan B, Smith JW. A redox site involved in integrin activation. J Biol Chem. 2000 Dec 22;275(51):39964-72. doi: 10.1074/jbc.M007041200. PMID:10993900 doi:http://dx.doi.org/10.1074/jbc.M007041200
Diseases and relevance:
- Estevez B, Shen B, Du X. Targeting integrin and integrin signaling in treating thrombosis. Arterioscler Thromb Vasc Biol. 2015 Jan;35(1):24-9. doi:, 10.1161/ATVBAHA.114.303411. Epub 2014 Sep 25. PMID:25256236 doi:http://dx.doi.org/10.1161/ATVBAHA.114.303411
- Bledzka K, Smyth SS, Plow EF. Integrin alphaIIbbeta3: from discovery to efficacious therapeutic target. Circ Res. 2013 Apr 12;112(8):1189-200. doi: 10.1161/CIRCRESAHA.112.300570. PMID:23580774 doi:http://dx.doi.org/10.1161/CIRCRESAHA.112.300570
- Wang R, Shattil SJ, Ambruso DR, Newman PJ. Truncation of the cytoplasmic domain of beta3 in a variant form of Glanzmann thrombasthenia abrogates signaling through the integrin alpha(IIb)beta3 complex. J Clin Invest. 1997 Nov 1;100(9):2393-403. doi: 10.1172/JCI119780. PMID:9351872 doi:http://dx.doi.org/10.1172/JCI119780