We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Nuclear polyadenylated RNA-binding protein
From Proteopedia
(Difference between revisions)
m (Hrp1 moved to Nuclear polyadenylated RNA-binding protein: requested by Editor) |
|||
| Line 3: | Line 3: | ||
=Introduction= | =Introduction= | ||
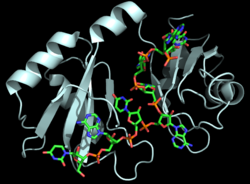
[[Image:Hrp1 fig1 cropped.png|250 px|right|thumb|Figure 1: Cartoon representation of the Hrp1-PEE complex. The RNA is shown as a stick model and is colored by element.]] | [[Image:Hrp1 fig1 cropped.png|250 px|right|thumb|Figure 1: Cartoon representation of the Hrp1-PEE complex. The RNA is shown as a stick model and is colored by element.]] | ||
| - | Hrp1 is a [https://en.wikipedia.org/wiki/Polyadenylation polyadenylation] factor found in ''Saccharomyces cerevisiae'' (yeast) <ref name="GM3H">PMID: 16794580</ref>. Hrp1 specifically recognizes and binds to an RNA sequence in the [https://en.wikipedia.org/wiki/Three_prime_untranslated_region 3'UTR] of the [https://en.wikipedia.org/wiki/Messenger_RNA messenger RNA (mRNA)] upstream from the cleavage site called the polyadenylation enhancement element (PEE) (Figure 1) <ref name="GM3H"/>. Upon binding to the RNA, Hrp1 helps recruit additional proteins necessary for the cleavage and polyadenylation of the RNA molecule <ref name="GM3H"/>. Although Hrp1 shares several common features with other RNA-binding proteins, the unique structural features of the Hrp1-PEE complex reveals the mechanism by which Hrp1 is able to recognize and bind to its specific RNA sequence at the atomic level <ref name="GM3H"/>. Hrp1 was discovered when Cleavage Factor I (CF I) was purified and separated into its two components, CF IA and CF IB. CF IB is a single 73 kDa polypeptide. The polypeptide was digested and two tryptic peptides were obtained for sequencing. The sequences were aligned via a database, and Hrp1 was determined to be a perfect match. Hrp1 of CF IB interacts with Rna14 and Rna15 of CF IA<ref name="RNA15"/> to form a protein complex that aids in cleavage and polyadenylation of pre-mRNA and transport of mature mRNA from the nucleus<ref name="KHSZ">PMID: 9334319</ref>. | + | '''Nuclear polyadenylated RNA-binding proteins''' (NAB) are yeast proteins which may be required for packaging pre-mRNAs into ribonucleoprotein structures amenable to efficient nuclear RNA processing<ref>PMID:7962083</ref>.<br /> |
| + | *'''Nab2''' is essential for cell viability<ref>PMID:8474438</ref>. <br /> | ||
| + | *'''Nab3''' interacts with the nascent RNA transcript and [[RNA polymerase]] II<ref>PMID:23192344</ref>. <br /> | ||
| + | *'''Hrp1''' or '''Nab4''' is a [https://en.wikipedia.org/wiki/Polyadenylation polyadenylation] factor found in ''Saccharomyces cerevisiae'' (yeast) <ref name="GM3H">PMID: 16794580</ref>. Hrp1 specifically recognizes and binds to an RNA sequence in the [https://en.wikipedia.org/wiki/Three_prime_untranslated_region 3'UTR] of the [https://en.wikipedia.org/wiki/Messenger_RNA messenger RNA (mRNA)] upstream from the cleavage site called the polyadenylation enhancement element (PEE) (Figure 1) <ref name="GM3H"/>. Upon binding to the RNA, Hrp1 helps recruit additional proteins necessary for the cleavage and polyadenylation of the RNA molecule <ref name="GM3H"/>. Although Hrp1 shares several common features with other RNA-binding proteins, the unique structural features of the Hrp1-PEE complex reveals the mechanism by which Hrp1 is able to recognize and bind to its specific RNA sequence at the atomic level <ref name="GM3H"/>. Hrp1 was discovered when Cleavage Factor I (CF I) was purified and separated into its two components, CF IA and CF IB. CF IB is a single 73 kDa polypeptide. The polypeptide was digested and two tryptic peptides were obtained for sequencing. The sequences were aligned via a database, and Hrp1 was determined to be a perfect match. Hrp1 of CF IB interacts with Rna14 and Rna15 of CF IA<ref name="RNA15"/> to form a protein complex that aids in cleavage and polyadenylation of pre-mRNA and transport of mature mRNA from the nucleus<ref name="KHSZ">PMID: 9334319</ref>. | ||
Revision as of 09:20, 20 January 2019
| |||||||||||
3D Structures of nuclear polyadenylated RNA-binding protein
Updated on 20-January-2019
References
- ↑ Wilson SM, Datar KV, Paddy MR, Swedlow JR, Swanson MS. Characterization of nuclear polyadenylated RNA-binding proteins in Saccharomyces cerevisiae. J Cell Biol. 1994 Dec;127(5):1173-84. PMID:7962083
- ↑ Anderson JT, Wilson SM, Datar KV, Swanson MS. NAB2: a yeast nuclear polyadenylated RNA-binding protein essential for cell viability. Mol Cell Biol. 1993 May;13(5):2730-41. PMID:8474438
- ↑ Loya TJ, O'Rourke TW, Reines D. Yeast Nab3 protein contains a self-assembly domain found in human heterogeneous nuclear ribonucleoprotein-C (hnRNP-C) that is necessary for transcription termination. J Biol Chem. 2013 Jan 25;288(4):2111-7. doi: 10.1074/jbc.M112.430678. Epub 2012, Nov 28. PMID:23192344 doi:http://dx.doi.org/10.1074/jbc.M112.430678
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 4.15 4.16 4.17 4.18 4.19 4.20 4.21 Perez-Canadillas JM. Grabbing the message: structural basis of mRNA 3'UTR recognition by Hrp1. EMBO J. 2006 Jul 12;25(13):3167-78. Epub 2006 Jun 22. PMID:16794580
- ↑ 5.0 5.1 5.2 5.3 5.4 Leeper TC, Qu X, Lu C, Moore C, Varani G. Novel protein-protein contacts facilitate mRNA 3'-processing signal recognition by Rna15 and Hrp1. J Mol Biol. 2010 Aug 20;401(3):334-49. Epub 2010 Jun 19. PMID:20600122 doi:10.1016/j.jmb.2010.06.032
- ↑ Kessler MM, Henry MF, Shen E, Zhao J, Gross S, Silver PA, Moore CL. Hrp1, a sequence-specific RNA-binding protein that shuttles between the nucleus and the cytoplasm, is required for mRNA 3'-end formation in yeast. Genes Dev. 1997 Oct 1;11(19):2545-56. PMID:9334319
- ↑ Clery A, Blatter M, Allain FH. RNA recognition motifs: boring? Not quite. Curr Opin Struct Biol. 2008 Jun;18(3):290-8. doi: 10.1016/j.sbi.2008.04.002. PMID:18515081 doi:http://dx.doi.org/10.1016/j.sbi.2008.04.002
Proteopedia Page Contributors and Editors (what is this?)
Cory A. Wuerch, Matthew Douglas Moore, Savannah Davis, Michal Harel, Jaime Prilusky