Tropomyosin
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
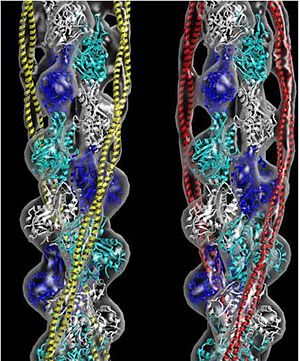
[[Image:B Lehman1.jpg | thumb | 300 x 430px | left | alt text | '''Tropomyosin''' (seen in yellow and red) wrapped around actin filaments, which are EM reconstructions with G-actin ribbion structures filling in the EM structure. (Picture generated from William Lehman's [http://www.bumc.bu.edu/phys-biophys/research/filhel/ Website]) ]] | [[Image:B Lehman1.jpg | thumb | 300 x 430px | left | alt text | '''Tropomyosin''' (seen in yellow and red) wrapped around actin filaments, which are EM reconstructions with G-actin ribbion structures filling in the EM structure. (Picture generated from William Lehman's [http://www.bumc.bu.edu/phys-biophys/research/filhel/ Website]) ]] | ||
==Function== | ==Function== | ||
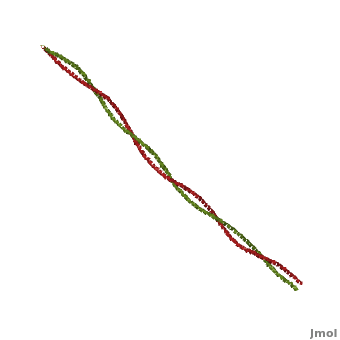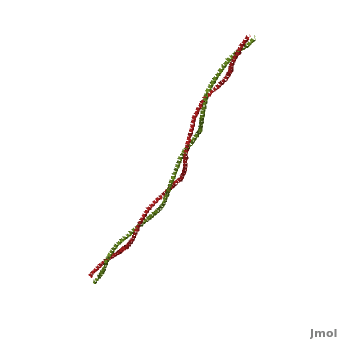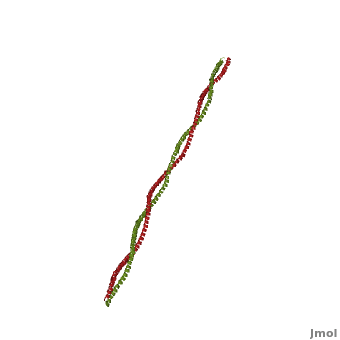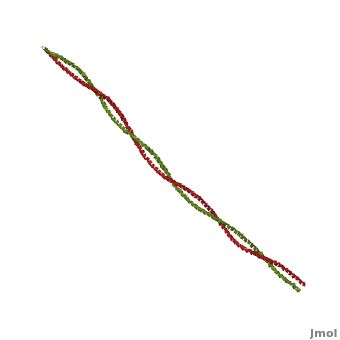
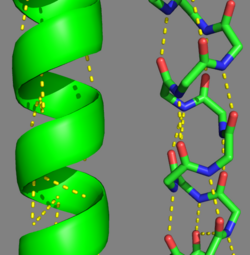
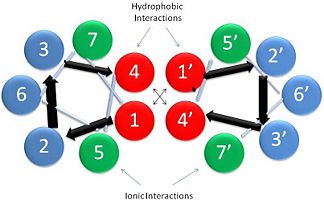
| - | '''[[Tropomyosin]] (TM)''' is an [[actin]] binding protein, which consists of a coiled-coil dimer (see left) and forms a polymer along the length of actin by a head-to-tail overlap along the major grove of actin (see down & left)<ref name="Gunning">Tropomyosins. I. Gunning, Peter, 1950- II. Series.[DNLM: 1. Tropomyosin. W1 AD559 v.644 2008 / WE 500 T856 2008]</ref>. The head-to-tail overlap allows flexibility between the tropomyosin dimers so it will lay unstrained along the filament<ref name="Gunning"/>. Each tropomyosin molecule spans seven actin monomers within a filament and lays N- to C- terminally from actin's pointed to barbed end<ref name="Frye">PMID:20465283</ref>. The 284 amino acid helix has a length of 420 Angstroms and has a molecular weight around 65-70 kilodaltons (vertebrate tropomyosin)<ref name="Gunning"/><ref name="Whitby">PMID:10651038</ref>. A few of tropomyosin's characteristics as an actin binding protein includes regulation, stabilization and recruitment. | + | '''[[Tropomyosin]] (TM)''' is an [[actin]] binding protein, which consists of a coiled-coil dimer (see left) and forms a polymer along the length of actin by a head-to-tail overlap along the major grove of actin (see down & left)<ref name="Gunning">Tropomyosins. I. Gunning, Peter, 1950- II. Series.[DNLM: 1. Tropomyosin. W1 AD559 v.644 2008 / WE 500 T856 2008]</ref>. See [[Coiled coil]]. The head-to-tail overlap allows flexibility between the tropomyosin dimers so it will lay unstrained along the filament<ref name="Gunning"/>. Each tropomyosin molecule spans seven actin monomers within a filament and lays N- to C- terminally from actin's pointed to barbed end<ref name="Frye">PMID:20465283</ref>. The 284 amino acid helix has a length of 420 Angstroms and has a molecular weight around 65-70 kilodaltons (vertebrate tropomyosin)<ref name="Gunning"/><ref name="Whitby">PMID:10651038</ref>. A few of tropomyosin's characteristics as an actin binding protein includes regulation, stabilization and recruitment. |
<br /> | <br /> | ||
Its role in muscle mechanics has been well established as it is a major regulatory component of the contractile apparatus, but its role in non-muscle systems is becoming evermore clear. In mammals, there are at least 40 known isoforms, which are generated by alternative splicing of multiple genes<ref name="Gunning"/><ref name="Frye"/>. These isoforms in non-muscle systems contribute to actin's stability by increasing filament rigidity and protecting the filament from actin severing proteins, like [[gelsolin]] or [http://en.wikipedia.org/wiki/Cofilin cofilin]. The different isoforms also aid in recruitment of various proteins, including myosin (a family of molecular motors)<ref name="Clayton"/><ref name="Stark"/>. The reason for tropomyosin's diversity is not well known, but it is thought to exist to function at different developmental stages in some species as well as function in specific cells of higher multicellular organisms<ref name="Gunning"/>. | Its role in muscle mechanics has been well established as it is a major regulatory component of the contractile apparatus, but its role in non-muscle systems is becoming evermore clear. In mammals, there are at least 40 known isoforms, which are generated by alternative splicing of multiple genes<ref name="Gunning"/><ref name="Frye"/>. These isoforms in non-muscle systems contribute to actin's stability by increasing filament rigidity and protecting the filament from actin severing proteins, like [[gelsolin]] or [http://en.wikipedia.org/wiki/Cofilin cofilin]. The different isoforms also aid in recruitment of various proteins, including myosin (a family of molecular motors)<ref name="Clayton"/><ref name="Stark"/>. The reason for tropomyosin's diversity is not well known, but it is thought to exist to function at different developmental stages in some species as well as function in specific cells of higher multicellular organisms<ref name="Gunning"/>. | ||
Revision as of 11:31, 22 January 2019
| |||||||||||
3D structures of Tropomyosin
Updated on 22-January-2019
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 1.24 Tropomyosins. I. Gunning, Peter, 1950- II. Series.[DNLM: 1. Tropomyosin. W1 AD559 v.644 2008 / WE 500 T856 2008]
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Frye J, Klenchin VA, Rayment I. Structure of the tropomyosin overlap complex from chicken smooth muscle: insight into the diversity of N-terminal recognition . Biochemistry. 2010 Jun 15;49(23):4908-20. PMID:20465283 doi:10.1021/bi100349a
- ↑ 3.0 3.1 3.2 Whitby FG, Phillips GN Jr. Crystal structure of tropomyosin at 7 Angstroms resolution. Proteins. 2000 Jan 1;38(1):49-59. PMID:10651038
- ↑ 4.0 4.1 4.2 Clayton JE, Sammons MR, Stark BC, Hodges AR, Lord M. Differential regulation of unconventional fission yeast myosins via the actin track. Curr Biol. 2010 Aug 24;20(16):1423-31. Epub 2010 Aug 12. PMID:20705471 doi:10.1016/j.cub.2010.07.026
- ↑ 5.0 5.1 Stark BC, Sladewski TE, Pollard LW, Lord M. Tropomyosin and myosin-II cellular levels promote actomyosin ring assembly in fission yeast. Mol Biol Cell. 2010 Mar 15;21(6):989-1000. Epub 2010 Jan 28. PMID:20110347 doi:10.1091/mbc.E09-10-0852
- ↑ 6.0 6.1 Drees B, Brown C, Barrell BG, Bretscher A. Tropomyosin is essential in yeast, yet the TPM1 and TPM2 products perform distinct functions. J Cell Biol. 1995 Feb;128(3):383-92. PMID:7844152
- ↑ 7.0 7.1 7.2 7.3 7.4 Lehman W, Galinska-Rakoczy A, Hatch V, Tobacman LS, Craig R. Structural basis for the activation of muscle contraction by troponin and tropomyosin. J Mol Biol. 2009 May 15;388(4):673-81. Epub 2009 Mar 31. PMID:19341744 doi:10.1016/j.jmb.2009.03.060
- ↑ 8.0 8.1 Tyska MJ, Warshaw DM. The myosin power stroke. Cell Motil Cytoskeleton. 2002 Jan;51(1):1-15. PMID:11810692 doi:10.1002/cm.10014
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Gregory Hoeprich, Jaime Prilusky, David Canner, Joel L. Sussman