Journal:Acta Cryst F:S2053230X19001213
From Proteopedia
(Difference between revisions)

| Line 8: | Line 8: | ||
<scene name='80/806392/Cv/8'>Overall structure of MtDPPS</scene>. The two monomers in an asymmetric unit of the MtDPPS crystal are shown as ribbon diagrams. The β-strands are named A-F and the α-helices numbered 1-7 from N to C terminus. They are colored yellow/red for one subunit and magenta/cyan for the other. | <scene name='80/806392/Cv/8'>Overall structure of MtDPPS</scene>. The two monomers in an asymmetric unit of the MtDPPS crystal are shown as ribbon diagrams. The β-strands are named A-F and the α-helices numbered 1-7 from N to C terminus. They are colored yellow/red for one subunit and magenta/cyan for the other. | ||
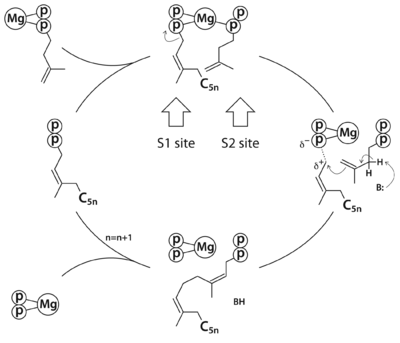
| - | <scene name='80/806392/Cv1/6'>Binding modes of substrate analogues</scene>. The MtDPPS dimer is superimposed on itself with the two polypeptide chains switched. The protein is colored cyan/green in one dimer and pink/yellow in the other, and so are the side chains and the ligands, which are shown as stick models. Mg and water molecules are shown as spheres, and the coordinate bonds as dashed lines. Location of the S1 and S2 site as well as the nearby helices α1/α2 and strand βB are also indicated. When the S1 and S2 substrates and Mg are properly bound for catalysis, the <scene name='80/806392/Cv1/9'>Asp76 side chain not only binds directly to Mg but also to a coordinating water molecule</scene>. The <scene name='80/806392/Cv1/10'>same water is hydrogen bonded to the side chain of Arg292*, which also binds to the other Mg-bound water</scene>. In the absence of both the S2 substrate and Mg, <scene name='80/806392/Cv1/11'>Arg292* turns to bind directly to Asp76</scene>, which is no longer engaged in Mg-coordination. The side chain of Arg292* binds to the β-phosphate of the S1 substrate in this conformation, and in the other it is also close to the β-phosphate of the S2 substrate. | + | <scene name='80/806392/Cv1/6'>Binding modes of substrate analogues</scene>. The MtDPPS dimer is superimposed on itself with the two polypeptide chains switched. The protein is colored cyan/green in one dimer and pink/yellow in the other, and so are the side chains and the ligands, which are shown as stick models. Mg and water molecules are shown as spheres, and the coordinate bonds as dashed lines. Location of the S1 and S2 site as well as the nearby helices α1/α2 and strand βB are also indicated. When the S1 and S2 substrates and Mg are properly bound for catalysis, the <scene name='80/806392/Cv1/9'>Asp76 side chain not only binds directly to Mg but also to a coordinating water molecule</scene>. The <scene name='80/806392/Cv1/10'>same water is hydrogen bonded to the side chain of Arg292*, which also binds to the other Mg-bound water</scene>. In the absence of both the S2 substrate and Mg, <scene name='80/806392/Cv1/11'>Arg292* turns to bind directly to Asp76</scene>, which is no longer engaged in Mg-coordination. The <scene name='80/806392/Cv1/12'>side chain of Arg292* binds to the β-phosphate of the S1 substrate in this conformation</scene>, and in the other it is also close to the β-phosphate of the S2 substrate. |
<scene name='80/806392/Cv1/8'>Mg coordination site</scene>. | <scene name='80/806392/Cv1/8'>Mg coordination site</scene>. | ||
Revision as of 14:58, 29 January 2019
| |||||||||||
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.