Aminoacyl tRNA Synthetase
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
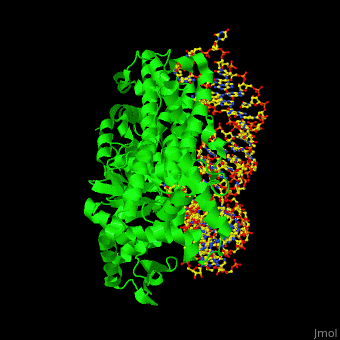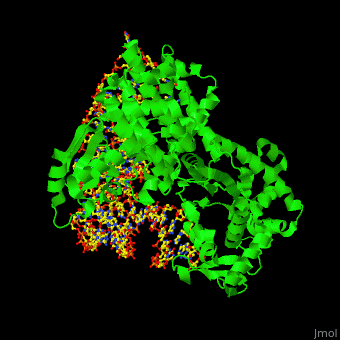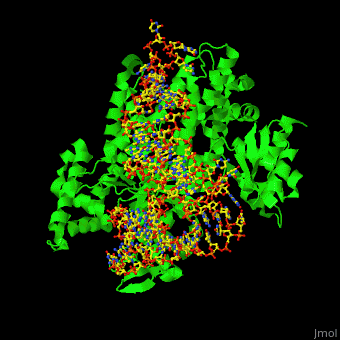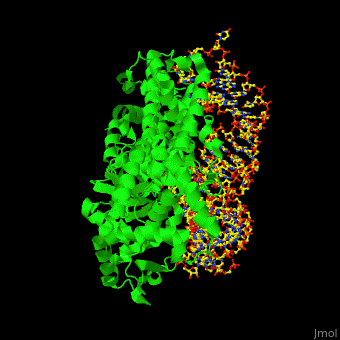
'''Aminoacyl tRNA synthetase''' (aaRS) or '''tRNA ligase''' catalyzes the esterification of a specific amino acid to its cognate tRNA to form an aminoacyl-tRNA. The amino acid is transferred by the ribosome from the aminoacylated-tRNA onto a growing polypeptide chain. Class I of aaRS is a monomer or dimer, it has 2 highly conserved sequence motifs and it aminoacylates at the 2’-OH of an adenosine nucleotide. Class II of aaRS is a dimer or tetramer, it has 3 highly conserved sequence motifs and it aminoacylates at the 3’-OH of an adenosine nucleotide. CP1 domain of RS edits a mischarged aa-tRNA. Some of the crystal structures are complexes of the RS with their reactant analog: amino acid-sulfamoyl adenine (aa-SA).<ref>PMID:8422978</ref>. | '''Aminoacyl tRNA synthetase''' (aaRS) or '''tRNA ligase''' catalyzes the esterification of a specific amino acid to its cognate tRNA to form an aminoacyl-tRNA. The amino acid is transferred by the ribosome from the aminoacylated-tRNA onto a growing polypeptide chain. Class I of aaRS is a monomer or dimer, it has 2 highly conserved sequence motifs and it aminoacylates at the 2’-OH of an adenosine nucleotide. Class II of aaRS is a dimer or tetramer, it has 3 highly conserved sequence motifs and it aminoacylates at the 3’-OH of an adenosine nucleotide. CP1 domain of RS edits a mischarged aa-tRNA. Some of the crystal structures are complexes of the RS with their reactant analog: amino acid-sulfamoyl adenine (aa-SA).<ref>PMID:8422978</ref>. | ||
*For '''leucine-RS''' details see [[Leucyl-tRNA Synthetase]]. | *For '''leucine-RS''' details see [[Leucyl-tRNA Synthetase]]. | ||
| - | *For '''pyrrolysyl-RS''' of '''pyrrolysine-tRNA ligase''' details see [[Pyrrolysyl-tRNA synthetase]]. | + | *For '''pyrrolysyl-RS''' of '''pyrrolysine-tRNA ligase''' details see [[Pyrrolysyl-tRNA synthetase]], [[Pyrrolysine]]. |
</StructureSection> | </StructureSection> | ||
Revision as of 09:11, 5 February 2019
| |||||||||||
3D Structures of Aminoacyl tRNA synthetase
Updated on 05-February-2019
References
- ↑ Cavarelli J, Moras D. Recognition of tRNAs by aminoacyl-tRNA synthetases. FASEB J. 1993 Jan;7(1):79-86. PMID:8422978
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Joel L. Sussman, Ann Taylor