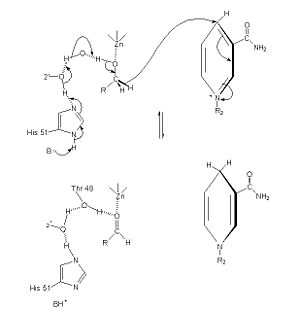
Alcohol dehydrogenase
From Proteopedia
(Difference between revisions)
| Line 74: | Line 74: | ||
For additional information, see: [[Carbohydrate Metabolism]]<br /> | For additional information, see: [[Carbohydrate Metabolism]]<br /> | ||
| - | == 3D Structures of Alcohol dehydrogenase== | ||
| - | |||
| - | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| - | {{#tree:id=OrganizedByTopic|openlevels=0| | ||
| - | |||
| - | *ADH I | ||
| - | |||
| - | **[[3jv7]] – RrADH I – ''Rhodococcus rubber''<br /> | ||
| - | **[[2vna]] - hADH I catalytic domain - human<br /> | ||
| - | **[[4w6z]] – yADH I – yeast<br /> | ||
| - | **[[4eex]] – LlADH I – ''Lactococcus lactis''<br /> | ||
| - | **[[4eez]] – LlADH I (mutant) | ||
| - | |||
| - | *ADH I binary complex | ||
| - | |||
| - | **[[1u3t]] – hADH I α chain + inhibitor<br /> | ||
| - | **[[1hsz]], [[1hdz]], [[3hud]] - hADH I β chain + NAD<br /> | ||
| - | **[[1u3w]] - hADH I γ chain + inhibitor<br /> | ||
| - | **[[1ht0]] - hADH I γ chain (mutant) + NAD<br /> | ||
| - | **[[6c49]] – ADH I + Zn – ''Acinetobacter baumannii''<br /> | ||
| - | |||
| - | *ADH I ternary complex | ||
| - | |||
| - | **[[2xaa]] – RrADH I + NAD + alcohol<br /> | ||
| - | **[[6ffx]] – RrADH I + Zn + NAD<br /> | ||
| - | **[[6ffz]], [[5o8h]], [[5o8q]], [[5o9d]], [[5o9f]], [[6fg0]], [[5od3]] – RrADH I (mutant) + Zn + NAD<br /> | ||
| - | **[[3fx4]] – pADH I + NADP + inhibitor – pig<br /> | ||
| - | **[[4w6z]] – yADH I + Zn + NAD derivative<br /> | ||
| - | **[[5env]] – yADH I + Zn + NAD + alcohol<br /> | ||
| - | **[[2w98]], [[2w4q]] – hADH I catalytic domain + NADP + inhibitor<br /> | ||
| - | **[[1hso]] - hADH I α chain + NAD + pyrazole derivative<br /> | ||
| - | **[[1hdx]] - hADH I β chain + NAD + alcohol<br /> | ||
| - | **[[1u3u]], [[1u3v]] - hADH I β chain + inhibitor<br /> | ||
| - | **[[1deh]], [[1hdy]] - hADH I β chain + NAD + pyrazole derivative<br /> | ||
| - | **[[1htb]] - hADH I β3 chain + NAD + pyrazole derivative | ||
| - | |||
| - | *ADH II | ||
| - | |||
| - | **[[3owo]] – ZmADH II iron-dependent – ''Zymomonas mobilis'' | ||
| - | |||
| - | *ADH II complex | ||
| - | |||
| - | **[[3ox4]] - ZmADH II iron-dependent + NAD<br /> | ||
| - | **[[3cos]] - hADH II + NAD + Zn<br /> | ||
| - | **[[1e3e]] – mADH II + NADH – mouse<br /> | ||
| - | **[[1e3l]] - mADH II (mutant) + NADH<br /> | ||
| - | **[[1e3i]] - mADH II + NADH + inhibitor<br /> | ||
| - | **[[5yln]] – ADH II + Zn – ''Streptococcus pneumoniae''<br /> | ||
| - | |||
| - | *ADH III | ||
| - | |||
| - | **[[1m6h]], [[1m6w]], [[1teh]] - hADH III χ chain<br /> | ||
| - | **[[2fze]] - hADH III χ chain + ADP-ribose<br /> | ||
| - | **[[2fzw]], [[1mp0]] - hADH III χ chain + NAD<br /> | ||
| - | **[[1mc5]] – hADH III χ chain + glutathione + NADH<br /> | ||
| - | **[[1ma0]] - hADH III χ chain + dodecanoic acid + NAD<br /> | ||
| - | **[[3qj5]] - hADH III χ chain + inhibitor + NAD<br /> | ||
| - | **[[4dl9]], [[4dlb]] – tADH III + NAD – tomato<br /> | ||
| - | **[[4dla]] – tADH III<br /> | ||
| - | **[[5mln]] – ADH III + NADPH – ''Candida magnoliae''<br /> | ||
| - | **[[5yat]] – ADH III + Zn – ''Komagsaataella phaffii''<br /> | ||
| - | |||
| - | *ADH IV | ||
| - | |||
| - | **[[1ye3]], [[8adh]], [[5adh]], [[4xd2]] - hoADH IV e chain – horse<br /> | ||
| - | **[[1qlj]] - hoADH IV e chain (mutant) <br /> | ||
| - | **[[3iv7]] – ADH IV – ''Corynebacterium glutamicum'' | ||
| - | |||
| - | *ADH IV binary complex | ||
| - | |||
| - | **[[2jhf]], [[2jhg]], [[1het]], [[1heu]], [[1hf3]], [[1ee2]], [[2oxi]], [[2ohx]], [[6adh]] - hoADH IV e chain + NAD<br /> | ||
| - | **[[1adb]], [[1adc]], [[1adf]], [[1adg]], [[7adh]], [[5vn1]] - hoADH IV e chain + NAD derivative<br /> | ||
| - | **[[1mgo]], [[1ju9]], [[1qlh]], [[1a72]] - hoADH IV e chain (mutant) + NAD<br /> | ||
| - | **[[1d1s]], [[1agn]] – hADH IV σ chain + NAD<br /> | ||
| - | **[[1d1t]] - hADH IV σ chain (mutant) + NAD | ||
| - | |||
| - | *ADH IV ternary complex | ||
| - | |||
| - | **[[3oq6]], [[1qv6]], [[1qv7]], [[1a71]], [[1axe]], [[1axg]], [[4nfh]], [[4nfs]], [[4ng5]], [[5kje]], [[5kjc]], [[5kj6]], [[5kj1]], [[5kcz]], [[5kcp]], [[5cdt]], [[5cds]], [[5cdg]] – hoADH IV e chain (mutant) + NAD + alcohol<br /> | ||
| - | **[[4dwv]], [[4dxh]], [[5kjf]] - hoADH IV e chain + NAD + alcohol<br /> | ||
| - | **[[1p1r]], [[1ldy]], [[1lde]] - hoADH IV e chain + NADH + formamide derivative<br /> | ||
| - | **[[1n92]] - hoADH IV e chain + NAD + pyrazole derivative<br /> | ||
| - | **[[1bto]], [[3bto]] - hoADH IV e chain + NADH + butylthiolane derivative<br /> | ||
| - | **[[1n8k]] - hoADH IV e chain (mutant) + NAD + pyrazole<br /> | ||
| - | **[[1mg0]], [[1hld]] - hoADH IV e chain + NAD + alcohol<br /> | ||
| - | **[[5vj5]] - hoADH IV e chain + Zn + phenanthroline<br /> | ||
| - | **[[5vjg]] - hoADH IV e chain + Zn + bipyridine<br /> | ||
| - | **[[5vkr]] - hoADH IV e chain + Zn + ADPR<br /> | ||
| - | **[[5vl0]] - hoADH IV e chain + Zn + formamide derivative <br /> | ||
| - | **[[6cy3]], [[6cxx]] - hoADH IV e chain (mutant) + Zn + dephosphoCoA<br /> | ||
| - | **[[5cdu]] - hoADH IV e chain (mutant) + Zn + NAD + pyrazole<br /> | ||
| - | |||
| - | *ADH | ||
| - | |||
| - | **[[1a4u]] – SlADH – ''Scaptodrosophila lebanonensis''<br /> | ||
| - | **[[3my7]] – ADH ACDH domain – ''Vibrio parahaemolyticus''<br /> | ||
| - | **[[3meq]] – ADH – ''Brucella suis''<br /> | ||
| - | **[[3l4p]] – ADH – ''Desulfovibrio gigas''<br /> | ||
| - | **[[1jvb]] - SsADH – ''Sulfolobus solfataricus''<br /> | ||
| - | **[[3i4c]], [[1nto]], [[1nvg]] – SsADH (mutant) <br /> | ||
| - | **[[3goh]] – ADH – ''Shewanella oneidensis''<br /> | ||
| - | **[[3gaz]] – ADH residues 2-334 – ''Novosphingobium aromaticivorans''<br /> | ||
| - | **[[2eih]] – ADH – ''Thermus thermophilus''<br /> | ||
| - | **[[1rjw]] – GsADH – ''Geobacillus stearothermophilus''<br /> | ||
| - | **[[1vj0]], [[1vhd]] – TmADH -''Thermotoga maritima''<br /> | ||
| - | **[[2eer]] – ADH – ''Sulfolobus tokodaii''<br /> | ||
| - | **[[3uog]] – ADH – ''Sinorhizobium meliloti''<br /> | ||
| - | **[[4bmn]] – ReADH - ''Ralstonia eutropha''<br /> | ||
| - | **[[4z6k]] – ADH - ''Moraxella''<br /> | ||
| - | |||
| - | *ADH binary complex | ||
| - | |||
| - | **[[3l77]], [[3tn7]] – ADH short-chain + NADP – ''Thermococcus sibiricus''<br /> | ||
| - | **[[1h2b]] – ADH + NAD – ''Aeropyrum pernix''<br /> | ||
| - | **[[1f8f]] – Benzyl-ADH + NAD – ''Acinetobacter calcoaceticus''<br /> | ||
| - | **[[1o2d]] - TmADH + NADP <br /> | ||
| - | **[[3ip1]] – TmADH + Cd<br /> | ||
| - | **[[1b16]], [[1b14]], [[1b15]] - SlADH + NAD derivative<br /> | ||
| - | **[[1cdo]] – ADH + NAD - cod<br /> | ||
| - | **[[1rhc]] – ADH F420-dependent +F420-acetone – ''Methanoculleus thermophilus''<br /> | ||
| - | **[[1agn]] – hADH (sigma) +NAD<br /> | ||
| - | **[[3pii]] – GsADH + butyramide<br /> | ||
| - | **[[3rj5]], [[3rj9]] – SlADH (mutant) + NAD<br /> | ||
| - | **[[3s1l]] – ReADH + Zn <br /> | ||
| - | **[[3jzd]] – ReADH + NAD<br /> | ||
| - | **[[4rqt]] - AtADH P + Zn – ''Arabidopsis thaliana'' <br /> | ||
| - | |||
| - | *ADH ternary complex | ||
| - | |||
| - | **[[1mg5]] – ADH + NADH + acetate – ''Drosophila melanogaster''<br /> | ||
| - | **[[1r37]] – SsADH + NAD + alcohol<br /> | ||
| - | **[[1sby]] – SlADH + NAD + alcohol<br /> | ||
| - | **[[1b2l]] - SlADH + NAD + cyclohexanone<br /> | ||
| - | **[[1llu]] - ADH + NAD + alcohol – ''Pseudomonas aeruginosa''<br /> | ||
| - | **[[3cv7]] – pADH + NAD + NAP<br /> | ||
| - | **[[3rf7]] – SoADH + NAD + Fe + Ni<br /> | ||
| - | **[[3s2e]] – ReADH + NAD + Zn<br /> | ||
| - | **[[3s2f]], [[3s2g]] – ReADH + NAD + Zn + furfural<br /> | ||
| - | **[[4gkv]] – EcADH + NAD + Zn + peptide – ''Escherichia coli''<br /> | ||
| - | **[[5vm2]] – EcADH + Mg + Zn<br /> | ||
| - | **[[4jji]], [[4gl4]], [[3uko]], [[4rqu]] - AtADH III + NAD + Zn <br /> | ||
| - | **[[4l0q]] - AtADH III (mutant) + NAD + Zn <br /> | ||
| - | **[[4cpd]] – TtADH + NAD + Zn<br /> | ||
| - | **[[6n7l]] – ADH + NAD + Zn – ''Elizabethkingia anophelis''<br /> | ||
| - | **[[6c76]] – TthADH + NADP + Fe – ''Thermococcus thioreducens''<br /> | ||
| - | **[[6c7l]] – TthADH + monophosphoadenosine diphosphate <br /> | ||
| - | **[[6c75]] – TthADH + NADP + Fe + monophosphoadenosine diphosphate <br /> | ||
| - | |||
| - | *NADP-dependent ADH | ||
| - | |||
| - | **[[1ped]] - CbADH – ''Clostridium beijerinckii''<br /> | ||
| - | **[[2b83]], [[1jqb]] – CbADH (mutant) <br /> | ||
| - | **[[2nvb]] - TbADH (mutant) – ''Thermoanaerobacter brockii''<br /> | ||
| - | **[[3ftn]], [[3fpc]], [[3fpl]], [[3fsr]] – ADH chimera<br /> | ||
| - | **[[1y9a]] - EhADH – ''Entamoeba histolytica''<br /> | ||
| - | **[[2oui]] – EhADH (mutant) <br /> | ||
| - | **[[1p0c]] – RpADH8 – ''Rana perezi'' <br /> | ||
| - | **[[4hfj]] – toADH – tobacco<br /> | ||
| - | **[[4gac]] - mADH | ||
| - | |||
| - | *NADP-dependent ADH complex | ||
| - | |||
| - | **[[1kev]] – CbADH + NADPH<br /> | ||
| - | **[[1bxz]] – CbADH catalytic domain + alcohol<br /> | ||
| - | **[[1ykf]] – TbADH + NADP<br /> | ||
| - | **[[3h4g]] – pADH + NADP<br /> | ||
| - | **[[1p0f]] – RpADH + NADP<br /> | ||
| - | **[[4hfm]] - toADH + NADP<br /> | ||
| - | **[[4hfn]] - toADH + NADP + coniferaldehyde<br /> | ||
| - | **[[4jbg]] - PaADH + Zn – ''Pyrobaculum aerophilum''<br /> | ||
| - | **[[4jbh]] - PaADH + Zn + Co<br /> | ||
| - | **[[4jbi]] - PaADH + NADP + Zn<br /> | ||
| - | **[[4bms]] – ReADH + NADPH<br /> | ||
| - | **[[4bmv]] – ADH + NADPH – ''Sphingobium yanoikuyae''<br /> | ||
| - | **[[5o98]] – ADH + NADPH – ''Catharanthus roseus''<br /> | ||
| - | **[[5yvm]] – bspADH + Mn + NADP derivative – brine sea pool<br /> | ||
| - | **[[5yvr]], [[5yvs]] – bspADH (mutant) + Mn + NADP <br /> | ||
| - | **[[5z2x]] – ADH + NADP – ''Kluyveromyces polyspora''<br /> | ||
| - | |||
| - | *R-specific ADH | ||
| - | |||
| - | **[[1nxq]], [[6h07]], [[6h1m]] - LbRADH – ''Lactobacillus brevis''<br /> | ||
| - | **[[1zk2]], [[1zk3]], [[6hlf]] - LbRADH (mutant)<br /> | ||
| - | **[[1zjy]], [[1zjz]], [[1zk0]], [[1zk1]] – LbRADH (mutant) + NADH + alcohol<br /> | ||
| - | **[[1zk4]] - LbRADH (mutant) + NADH + acetophenone<br /> | ||
| - | **[[4rf4]] – LkADH + Mg - ''Lactobacillus kefiri''<br /> | ||
| - | **[[4rf5]], [[4rf3]] – LkADH (mutant) + Mg <br /> | ||
| - | **[[4rf2]] – LkADH + Mg + NADP<br /> | ||
| - | |||
| - | *Specific alcohol ADH | ||
| - | |||
| - | **[[2cf5]], [[2cf6]] – Cinnamyl-AtADH <br /> | ||
| - | **[[1piw]], [[1q1n]], [[1ps0]] – Cinnamyl-yADH<br /> | ||
| - | **[[3two]] - Cinnamyl-ADH + NADP – ''Helicobacter pylori''<br /> | ||
| - | **[[1m2w]] – Mannitol-ADH – ''Pseudomonas fluorescens'' <br /> | ||
| - | **[[1w6s]] – Methanol-ADH – ''Methylobacterium extorquens''<br /> | ||
| - | **[[1yqx]] – Sinapyl-aADH II – aspen<br /> | ||
| - | **[[1yqd]] – Sinapyl-aADH II + NADP<br /> | ||
| - | **[[1bdb]] – Biphenyl dihydrodiol-ADH + NAD - ''Pseudomonas'' | ||
| - | |||
| - | *Quinohemoprotein ADH | ||
| - | |||
| - | **[[1kv9]], [[1yiq]] – PpQADH II + PQQ + heme – ''Pseudomonas putida''<br /> | ||
| - | **[[1kb0]] - QADH I + PQQ + heme – ''Comamonas testosteroni'' | ||
| - | |||
| - | *Quinone-dependent ADH | ||
| - | |||
| - | **[[4cvc]], [[4cvb]] – ADH + Zn + PQQ + propanoate – ''Pseudogluconobacter saccharoketogenes''<br /> | ||
| - | |||
| - | *Hydroxyacyl-CoA dehydrogenase (HADH) | ||
| - | |||
| - | **Short chain HADH | ||
| - | |||
| - | ***[[1so8]] – hSHCDH II – human<BR /> | ||
| - | ***[[3rqs]] - hSHCDH <BR /> | ||
| - | ***[[1f14]] - hSHCDH (mutant) | ||
| - | |||
| - | **Short chain HADH binary complex | ||
| - | |||
| - | ***[[1f12]] - hSHCDH (mutant) + hydroxybutyryl-CoA<BR /> | ||
| - | ***[[1f17]], [[1lsj]], [[1lso]] - hSHCDH (mutant) + NAD<BR /> | ||
| - | ***[[1zbq]] - hSHCDH IV + NAD<BR /> | ||
| - | ***[[1e3s]] - rSHCDH + NAD – rat | ||
| - | |||
| - | **Short chain HADH ternary complex | ||
| - | |||
| - | ***[[1u7t]] - hSHCDH II + inhibitor + NAD<BR /> | ||
| - | ***[[1f0y]] - hSHCDH + acetoacetyl-CoA + NAD<BR /> | ||
| - | ***[[1il0]], [[1m75]], [[1m76]] - hSHCDH (mutant) + acetoacetyl-CoA + NAD<BR /> | ||
| - | ***[[1e3w]] - rSHCDH + 3-keto-butyrate + NAD<BR /> | ||
| - | ***[[1e6w]] - rSHCDH + estradiol + NAD<BR /> | ||
| - | |||
| - | *Unspecified HADH | ||
| - | |||
| - | **[[1uay]] - HADH II – ''Thermus thermophilus''<BR /> | ||
| - | **[[1zej]], [[3ctv]] - HADH – ''Archaeoglobus fulgidus''<BR /> | ||
| - | **[[1zcj]] - rHADH <BR /> | ||
| - | **[[2x58]] - rHADH + CoA <BR /> | ||
| - | **[[2et6]] – HADH (mutant) – ''Candida tropicalis'' | ||
| - | }} | ||
==References== | ==References== | ||
Revision as of 08:32, 4 March 2019
| |||||||||||
Additional Resources
For additional information, see: Carbohydrate Metabolism
References
- ↑ Voet, et. al. Fundamentals of Biochemistry: 3rd Edition. Hoboken: Wiley & Sons, Inc, 2008.
- ↑ Voet, et. al. Fundamentals of Biochemistry: 3rd Edition. Hoboken: Wiley & Sons, Inc, 2008.
- ↑ Protein: Alcohol Dehydrogenase. The College of Saint Benedict and Saint John's University. 1 March 2010 < http://web.archive.org/web/20080307193453/http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm>
- ↑ Protein: Alcohol Dehydrogenase. The College of Saint Benedict and Saint John's University. 1 March 2010 < http://web.archive.org/web/20080307193453/http://www.users.csbsju.edu/~hjakubow/classes/rasmolchime/99ch331proj/alcoholdehydro/index.htm>
- ↑ Voet, et. al. Fundamentals of Biochemistry: 3rd Edition. Hoboken: Wiley & Sons, Inc, 2008.
- ↑ Dickinson FM, Monger GP. A study of the kinetics and mechanism of yeast alcohol dehydrogenase with a variety of substrates. Biochem J. 1973 Feb;131(2):261-70. PMID:4352908
- ↑ Dickinson FM, Monger GP. A study of the kinetics and mechanism of yeast alcohol dehydrogenase with a variety of substrates. Biochem J. 1973 Feb;131(2):261-70. PMID:4352908
- ↑ Bille V, Remacle J. Simple-kinetic descriptions of alcohol dehydrogenase after immobilization on tresyl-chloride-activated agarose. Eur J Biochem. 1986 Oct 15;160(2):343-8. PMID:3769934
- ↑ Dickinson FM, Monger GP. A study of the kinetics and mechanism of yeast alcohol dehydrogenase with a variety of substrates. Biochem J. 1973 Feb;131(2):261-70. PMID:4352908
- ↑ Blomstrand R, Ostling-Wintzell H, Lof A, McMartin K, Tolf BR, Hedstrom KG. Pyrazoles as inhibitors of alcohol oxidation and as important tools in alcohol research: an approach to therapy against methanol poisoning. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3499-503. PMID:115004
- ↑ Alcohol Dehydrogenase. Worthington Biochemical Corporation . 31 March 2010 < http://http://www.worthington-biochem.com/ADH/default.html>
- ↑ Alcohol Dehydrogenase.Worthington Biochemical Corporation . 31 March 2010 < http://http://www.worthington-biochem.com/ADH/default.html>
- ↑ Goihberg E, Dym O, Tel-Or S, Levin I, Peretz M, Burstein Y. A single proline substitution is critical for the thermostabilization of Clostridium beijerinckii alcohol dehydrogenase. Proteins. 2007 Jan 1;66(1):196-204. PMID:17063493 doi:10.1002/prot.21170
- ↑ Goihberg E, Dym O, Tel-Or S, Shimon L, Frolow F, Peretz M, Burstein Y. Thermal stabilization of the protozoan Entamoeba histolytica alcohol dehydrogenase by a single proline substitution. Proteins. 2008 Feb 7;. PMID:18260103 doi:10.1002/prot.21946
- ↑ Goihberg E, Peretz M, Tel-Or S, Dym O, Shimon L, Frolow F, Burstein Y. Biochemical and Structural Properties of Chimeras Constructed by Exchange of Cofactor-Binding Domains in Alcohol Dehydrogenases from Thermophilic and Mesophilic Microorganisms. Biochemistry. 2010 Feb 9. PMID:20102159 doi:10.1021/bi901730x
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, David Canner, Joel L. Sussman, David Birrer