We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Bacterial thiolase
From Proteopedia
(Difference between revisions)
| Line 145: | Line 145: | ||
a unique combination of these four sequence fingerprints <ref>PMID:24825023 </ref>. | a unique combination of these four sequence fingerprints <ref>PMID:24825023 </ref>. | ||
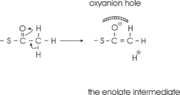
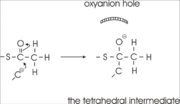
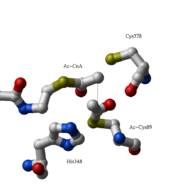
| - | [[Image:Fig4.png |left|thumb|Figure 3: Formation of the thioester enolate. A base is required for this proton abstraction. In thiolase this is Cys378. The oxyanion hole is formed by the Wat82-Asn316 diad and His348.]] | ||
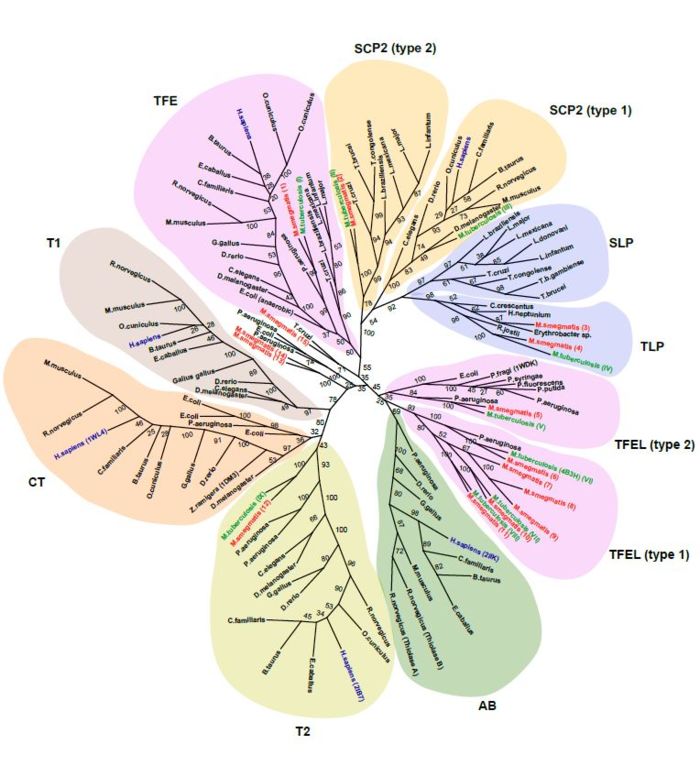
| - | [[Image:Figure-3-tuberculosis.jpeg|left|thumb|700px|Figure 6. This phylogenetic tree is based on a multiple sequence alignment of 130 sequences. Several clusters are found. This figure is figure 3 of a paper published in the journal | + | |
| + | [[Image:Figure-3-tuberculosis.jpeg|left|thumb|700px|Figure 6. This phylogenetic tree is based on a multiple sequence alignment of 130 sequences. Several clusters are found. This figure is figure 3 of a paper published in the journal Tuberculosis <ref>PMID:24825023 </ref>. It is reproduced with permission of the publisher]] | ||
</StructureSection> | </StructureSection> | ||
Revision as of 15:28, 9 March 2019
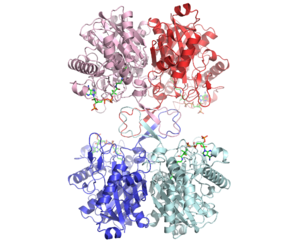
3D structure (1DM3) of the bacterial Zoogloea ramigera biosynthetic thiolase
| |||||||||||
Additional Resources
For additional information, see: Metabolic Disorders
3D structures of Thiolase
References
- ↑ Kiema TR, Thapa CJ, Laitaoja M, Schmitz W, Maksimainen MM, Fukao T, Rouvinen J, Janis J, Wierenga RK. The peroxisomal zebrafish SCP2-thiolase (type-1) is a weak transient dimer as revealed by crystal structures and native mass spectrometry. Biochem J. 2018 Dec 20. pii: BCJ20180788. doi: 10.1042/BCJ20180788. PMID:30573650 doi:http://dx.doi.org/10.1042/BCJ20180788
- ↑ Williams SF, Palmer MA, Peoples OP, Walsh CT, Sinskey AJ, Masamune S. Biosynthetic thiolase from Zoogloea ramigera. Mutagenesis of the putative active-site base Cys-378 to Ser-378 changes the partitioning of the acetyl S-enzyme intermediate. J Biol Chem. 1992 Aug 15;267(23):16041-3. PMID:1353760
- ↑ 3.0 3.1 Kursula P, Ojala J, Lambeir AM, Wierenga RK. The catalytic cycle of biosynthetic thiolase: a conformational journey of an acetyl group through four binding modes and two oxyanion holes. Biochemistry. 2002 Dec 31;41(52):15543-56. PMID:12501183
- ↑ 4.0 4.1 4.2 Merilainen G, Poikela VM, Kursula P, Wierenga RK. The thiolase reaction mechanism: the importance of Asn316 and His348 for stabilizing the enolate intermediate of the Claisen condensation. Biochemistry. 2009 Oct 20. PMID:19842716 doi:10.1021/bi901069h
- ↑ 5.0 5.1 Fukao T, Nguyen HT, Nguyen NT, Vu DC, Can NT, Pham AT, Nguyen KN, Kobayashi H, Hasegawa Y, Bui TP, Niezen-Koning KE, Wanders RJ, de Koning T, Nguyen LT, Yamaguchi S, Kondo N. A common mutation, R208X, identified in Vietnamese patients with mitochondrial acetoacetyl-CoA thiolase (T2) deficiency. Mol Genet Metab. 2010 May;100(1):37-41. Epub 2010 Jan 21. PMID:20156697 doi:10.1016/j.ymgme.2010.01.007
- ↑ Modis Y, Wierenga RK. Crystallographic analysis of the reaction pathway of Zoogloea ramigera biosynthetic thiolase. J Mol Biol. 2000 Apr 14;297(5):1171-82. PMID:10764581 doi:10.1006/jmbi.2000.3638
- ↑ Haapalainen AM, Merilainen G, Wierenga RK. The thiolase superfamily: condensing enzymes with diverse reaction specificities. Trends Biochem Sci. 2006 Jan;31(1):64-71. Epub 2005 Dec 13. PMID:16356722 doi:10.1016/j.tibs.2005.11.011
- ↑ Jiang C, Kim SY, Suh DY. Divergent evolution of the thiolase superfamily and chalcone synthase family. Mol Phylogenet Evol. 2008 Dec;49(3):691-701. Epub 2008 Sep 12. PMID:18824113 doi:10.1016/j.ympev.2008.09.002
- ↑ Anbazhagan P, Harijan RK, Kiema TR, Janardan N, Murthy MR, Michels PA, Juffer AH, Wierenga RK. Phylogenetic relationships and classification of thiolases and thiolase-like proteins of Mycobacterium tuberculosis and Mycobacterium smegmatis. Tuberculosis (Edinb). 2014 Jul;94(4):405-12. doi: 10.1016/j.tube.2014.03.003., Epub 2014 Apr 4. PMID:24825023 doi:http://dx.doi.org/10.1016/j.tube.2014.03.003
- ↑ Anbazhagan P, Harijan RK, Kiema TR, Janardan N, Murthy MR, Michels PA, Juffer AH, Wierenga RK. Phylogenetic relationships and classification of thiolases and thiolase-like proteins of Mycobacterium tuberculosis and Mycobacterium smegmatis. Tuberculosis (Edinb). 2014 Jul;94(4):405-12. doi: 10.1016/j.tube.2014.03.003., Epub 2014 Apr 4. PMID:24825023 doi:http://dx.doi.org/10.1016/j.tube.2014.03.003
Proteopedia Page Contributors and Editors (what is this?)
Rik Wierenga, Joel L. Sussman, Michal Harel, Satyan Sharma, David Canner