Glucuronidase
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
== Relevance == | == Relevance == | ||
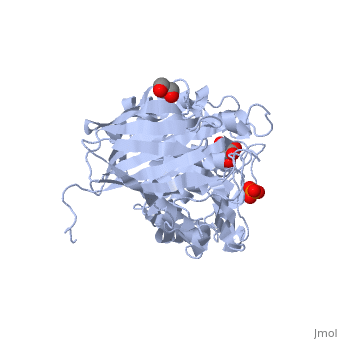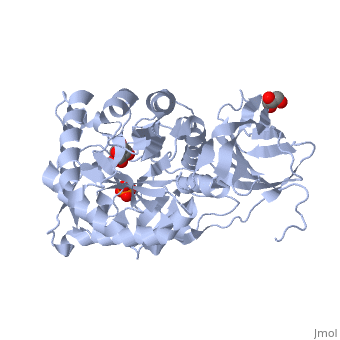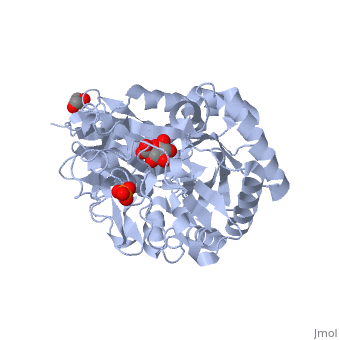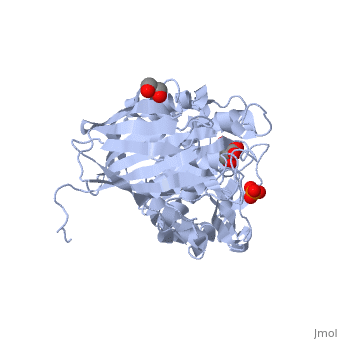
| - | The ''E. coli'' form of β-glucuronidase (<scene name='59/596447/E_coli_b-glucuronidase/1'>overall structure</scene>, PDB ID | + | The ''E. coli'' form of β-glucuronidase (<scene name='59/596447/E_coli_b-glucuronidase/1'>overall structure</scene>, PDB ID [[3lpf]]<ref>DOI:10.2210/pdb3lpf/pdb</ref>) is associated with the side effects seen with administration of the cancer chemotherapy drug CPT-11. This drug gets converted to SN38, a topoisomerase inhibitor, by the liver. The body adds a glucuronide group to this molecule (now SN38-G) to mark it for elimination, which partially occurs through the intestine. Once in the intestine, bacterial β-glucuronidase cleaves the glucuronide from the SN38-G, releasing the SN38 into the intestinal lumen. The released SN38 prevents cell division, compromising the epithelial lining of the intestines, a painful and dangerous side-effect of CPT-11 administration. |
Selective inhibition of bacterial β-glucuronidase is desired to alleviate this side-effect of CPT-11 treatment, hopefully without inhibiting the human form of the enzyme<ref>PMID:9829738</ref>. | Selective inhibition of bacterial β-glucuronidase is desired to alleviate this side-effect of CPT-11 treatment, hopefully without inhibiting the human form of the enzyme<ref>PMID:9829738</ref>. | ||
==Disease== | ==Disease== | ||
| - | Deficiencies in the human form of β-glucuronidase (<scene name='59/596447/Human_bglucuronidase/1'>overall structure</scene>, PDB ID | + | Deficiencies in the human form of β-glucuronidase (<scene name='59/596447/Human_bglucuronidase/1'>overall structure</scene>, PDB ID [[3hn3]]<ref>DOI:10.2210/pdb3hn3/pdb</ref>) is associated with a disease known as Sly Syndrome (AKA Mucopolysaccharidosis VII -- MPS VII). This disease is characterized by mental retardation, short stature, macrocephaly, and enlarged joints. As is commonly seen with genetic disorders, patients with this disease present a spectrum of symptom severity, but the disease is always ultimately fatal. |
== Structural highlights == | == Structural highlights == | ||
Revision as of 13:20, 1 April 2019
| |||||||||||
3D Structures of glucuronisidase
Updated on 01-April-2019
References
- ↑ Jain S, Drendel WB, Chen ZW, Mathews FS, Sly WS, Grubb JH. Structure of human beta-glucuronidase reveals candidate lysosomal targeting and active-site motifs. Nat Struct Biol. 1996 Apr;3(4):375-81. PMID:8599764
- ↑ Nagy T, Emami K, Fontes CM, Ferreira LM, Humphry DR, Gilbert HJ. The membrane-bound alpha-glucuronidase from Pseudomonas cellulosa hydrolyzes 4-O-methyl-D-glucuronoxylooligosaccharides but not 4-O-methyl-D-glucuronoxylan. J Bacteriol. 2002 Sep;184(17):4925-9. PMID:12169619
- ↑ doi: https://dx.doi.org/10.2210/pdb3lpf/pdb
- ↑ Sparreboom A, de Jonge MJ, de Bruijn P, Brouwer E, Nooter K, Loos WJ, van Alphen RJ, Mathijssen RH, Stoter G, Verweij J. Irinotecan (CPT-11) metabolism and disposition in cancer patients. Clin Cancer Res. 1998 Nov;4(11):2747-54. PMID:9829738
- ↑ doi: https://dx.doi.org/10.2210/pdb3hn3/pdb
- ↑ Michikawa M, Ichinose H, Momma M, Biely P, Jongkees S, Yoshida M, Kotake T, Tsumuraya Y, Withers SG, Fujimoto Z, Kaneko S. Structural and biochemical characterization of glycoside hydrolase family 79 beta-glucuronidase from Acidobacterium capsulatum. J Biol Chem. 2012 Apr 20;287(17):14069-77. Epub 2012 Feb 24. PMID:22367201 doi:http://dx.doi.org/10.1074/jbc.M112.346288